The materials we used for this experiment include four bouncy balls of almost identical specifications, stainless steel containers, water to heat or cool the bouncy balls, salt, an oven, a stove, a hot water tap, a refrigerator, a freezer, a thermometer, a video camera and tripod, a timer, tongs, tape, permanent markers, a measuring tape and Logger Pro to analyze data. First, we set up and prepared for the experiment by measuring a distance of four feet from the ground to partway up the wall, and marked the spot using tape and a permanent marker. Then we tested the control condition by bouncing the four bouncy balls twice each (for a total of eight trials) to gather our first set of data. Each bouncy ball was dropped as close to the four-foot line as possible and recorded using the video camera on a tripod. We dropped the bouncy balls in the same order each time: blue, orange, pink, and orange with a dot marked in permanent marker. After collecting this data, we began testing our experimental conditions. We tested the coldest sample first. We filled a bowl with water and salt, and put it in the freezer until it became very cold (about 15 minutes). Then we put the four bouncy balls in the water, set a timer for 5 minutes, and waited. When the timer went off, we tested the temperature of the water using the thermometer and recorded it. Then we used tongs to remove the bouncy balls, one at a time, in the proper order, and drop them from the four-foot mark while recording. After giving the bouncy balls time to warm up, we repeated the process using the same four bouncy balls to obtain a total of eight trials for that condition. We repeated this process for each of the other conditions. For the refrigerator trial, we simply placed a bowl of water in the refrigerator for approximately 15 minutes before adding the bouncy balls and repeating the process above. We also tested room temperature water, water placed in a 250 degree oven, water straight from a hot tap (or InstaHot), and water boiled on the stove. After all the trials had been done (seven conditions with eight trials each) we used Logger Pro to analyze the data. We had to set the origin in the video, and mark the point where the ball reached its maximum height. We recorded all of this data, calculated the averages for each trial, and created graphs. We also calculated uncertainty by subtracting the lowest data point for each condition from the highest data point in that condition and dividing by two. We used this information to create error bars.
|
Control: No Water |
|
|
|
|
|
Trial 1 |
Trial 2 |
Average |
|
Blue |
2.771 |
2.780 |
2.776 |
|
No-Dot Orange |
3.244 |
3.235 |
3.240 |
|
Pink |
3.290 |
3.152 |
3.221 |
|
Dot Orange |
3.182 |
3.192 |
3.187 |
|
|
|
|
3.106 |
|
|
|
|
|
|
Freezer and Salt: 25°F |
|
|
|
|
|
Trial 1 |
Trial 2 |
Average |
|
Blue |
2.822 |
3.081 |
2.952 |
|
No-Dot Orange |
3.095 |
2.761 |
2.928 |
|
Pink |
3.079 |
3.029 |
3.054 |
|
Dot Orange |
2.821 |
3.086 |
2.954 |
|
|
|
|
2.972 |
|
|
|
|
|
|
Refrigerator: 41°F |
|
|
|
|
|
Trial 1 |
Trial 2 |
Average |
|
Blue |
3.060 |
3.020 |
3.040 |
|
No-Dot Orange |
3.139 |
2.790 |
2.965 |
|
Pink |
3.020 |
2.809 |
2.915 |
|
Dot Orange |
2.993 |
3.068 |
3.031 |
|
|
|
|
2.987 |
|
|
|
|
|
|
Room Temperature: 59°F |
|
|
|
|
|
Trial 1 |
Trial 2 |
Average |
|
Blue |
3.084 |
3.109 |
3.097 |
|
No-Dot Orange |
2.981 |
3.128 |
3.055 |
|
Pink |
3.049 |
3.023 |
3.036 |
|
Dot Orange |
2.822 |
3.104 |
2.963 |
|
|
|
|
3.038 |
|
|
|
|
|
|
Oven, Cooled: 109°F |
|
|
|
|
|
Trial 1 |
Trial 2 |
Average |
|
Blue |
3.156 |
3.178 |
3.167 |
|
No-Dot Orange |
3.125 |
3.282 |
3.204 |
|
Pink |
2.818 |
2.891 |
2.855 |
|
Dot Orange |
3.200 |
3.121 |
3.161 |
|
|
|
|
3.096 |
|
Hot Tap: 140°F |
|
|
|
|
|
Trial 1 |
Trial 2 |
Average |
|
Blue |
3.044 |
3.199 |
3.122 |
|
No-Dot Orange |
3.163 |
3.259 |
3.211 |
|
Pink |
2.920 |
2.924 |
2.922 |
|
Dot Orange |
3.048 |
3.079 |
3.064 |
|
|
|
|
3.080 |
|
|
|
|
|
|
Stovetop: 201°F |
|
|
|
|
|
Trial 1 |
Trial 2 |
Average |
|
Blue |
3.201 |
2.428 |
2.815 |
|
No-Dot Orange |
3.209 |
3.364 |
3.287 |
|
Pink |
3.117 |
2.863 |
2.990 |
|
Dot Orange |
3.290 |
3.324 |
3.307 |
|
|
|
|
3.100 |
Averages:
|
Conditions: |
Temperature: (°F) |
Average Height: (ft) |
|
Control: No Water |
|
3.106 |
|
Freezer and Salt |
25 |
2.972 |
|
Refrigerator |
41 |
2.987 |
|
Room Temperature |
59 |
3.038 |
|
Oven, Cooled |
109 |
3.096 |
|
Hot Tap |
140 |
3.080 |
|
Stovetop |
201 |
3.100 |
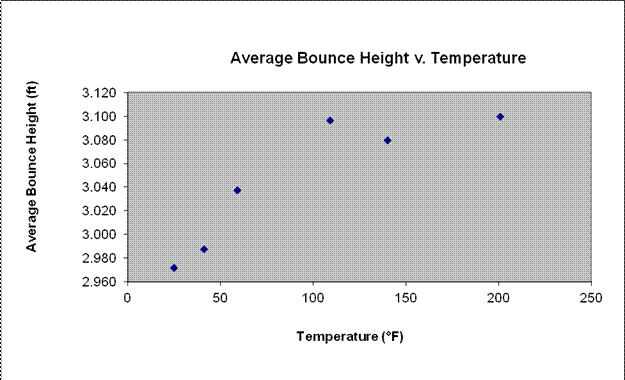
The results of our experiment demonstrated that, on average, at higher water temperatures, the bouncy balls bounced higher and at lower water temperatures, the bouncy balls bounced lower. The results suggested that the temperature of the water used to heat or cool a bouncy ball does, indeed, affect the bouncitivity of the bouncy balls. In our hypothesis, we predicted that as the temperature of the water increased, the bouncitivity would also increase in a linear manner. Our results indicated that the bouncitivity grew in a more logarithmic fashion. The fact that higher temperatures of water produce higher bouncing balls is supported by several ideas and theories. According to the Collision Theory, raising temperature brings about more collisions, therefore increasing the rate of reactions (in this case, bouncitivity). Our results are also supported by chemical kinetics, the idea that temperature usually has a major effect on the rate of chemical reaction. Molecules at a higher temperature usually have more thermal energy, increasing collision frequency. Interesting, the controlled trial, in which the bouncy balls were not soaked in water, had the highest bounce, on average, of any trial at 3.106 ft. This could have been for a few different reasons. It’s possible that some amount of water compromised the material of the bouncy ball, making it more dense or affecting the composition in some manner. It’s also possible that, since we did our cold trials first, cooling the bouncy balls to such a low temperature had some permanent damaging effect on the bouncy balls. Our only other piece of data that was not exactly consistent was the Oven, Cooled trial. This was our only trial where the method was slightly inconsistent. Instead of testing the temperature while the bowl was in the oven, as we did with the refrigerator, etc. in other trials, we took the bowl out of the oven to cool. We allowed it to cool off almost ten degrees before placing the bouncy balls in the bowl for five minutes, testing the temperature, and bouncing the balls like normal. This could have caused errors in that particular data point, accounting for is unexpectedly high average of 3.096 ft. Other sources of error may have included inconsistency in the person dropping the bouncy ball, inconsistency in the amount of time between removing the bouncy ball from the heated or cooled water and dropping it, and the extent to which the ball bounced straight up and down, or slightly to the left or right. Improvements or variations that could be made in the future include testing more temperatures, particularly high temperatures, using different sizes of bouncy balls, using different brands or compositions of bouncy balls, and testing on different surfaces.
Ament, Phil. "Super Ball History - Invention of the Super Ball." The Great Idea Finder - Celebrating the Spirit of Innovation. Troy MI: ©1997-2007 The Great Idea Finder, Mar. 2005. Web. 6 Nov. 2011. <http://www.ideafinder.com/history/inventions/superball.htm>.
Becker, Bob. "What Makes a Superball so Super?" Chem Matters Oct. 2005: 4-5. American Chemical Society. Web. 6 Nov. 2011. <http://portal.acs.org/preview/fileFetch/C/WPCP_008052/pdf/WPCP_008052.pdf>.
Blackman, Ben. "Superballs: What Makes Them Bounce?" Indianapublicmedia.org. Indiana Public Media | News and Information, Music, Arts and Community Events from WFIU and WTIU, 26 Aug. 2006. Web. 6 Nov. 2011. <http://indianapublicmedia.org/amomentofscience/superballs/>.
"Fads: It's a Bird, It's a Plane..." Time Magazine 22 Oct. 1965. Time, Inc. Web. 6 Nov. 2011. <http://www.time.com/time/subscriber/article/0,33009,941414,00.html>.
Harris, William. "How SuperBalls Work." HowStuffWorks.com. How Stuff Works. Web. 6 Nov. 2011. <http://people.howstuffworks.com/superball3.htm>.
O'Leary, Donal. "Sulphur." Ucc.ie. University College Cork, 2000. Web. 6 Nov. 2011. <http://www.ucc.ie/academic/chem/dolchem/html/elem016.html>.
"Wham-O History." Wham-O.com. Wham-O, Inc. Web. 6 Nov. 2011. <http://www.wham-o.com/history.html>.
"What Is the Super Ball Made Of?" Angelfire. Web. 6 Nov. 2011. <http://www.angelfire.com/extreme/whamo/madeof.htm>.
Related Websites
http://www.ideafinder.com/history/inventions/superball.htm
Useful in understanding background information about the history and invention of Superballs.
http://portal.acs.org/preview/fileFetch/C/WPCP_008052/pdf/WPCP_008052.pdf
Gives a good explanation of the science of Superballs.
http://indianapublicmedia.org/amomentofscience/superballs/
Explains why Superballs bounce and provides more science behind the bounce.
http://www.time.com/time/subscriber/article/0,33009,941414,00.html
Describes the popularity of Superballs and its history.
http://people.howstuffworks.com/superball3.htm
Yet another useful explanation of how Superballs work.
http://www.ucc.ie/academic/chem/dolchem/html/elem016.html
Background of some of the elements and components of the Superball.
http://www.wham-o.com/history.html
The official history of the Superball from the Wham-O website!
http://www.angelfire.com/extreme/whamo/madeof.htm
One last explanation of what the Superball is made of.