Correlation Between the Change in Potential Energy
of a Dropped Projectile and Displacement in Gelatin
by
Nick Miller, Nina Yang, Patreece Suen, and Margaret Campbell
INTRODUCTION .:. METHOD .:. RESULTS AND CALCULATIONS .:. CONCLUSION .:. BIBLIOGRAPHY .:. RELATED WEBSITES .:. Go Up
Gelatin is a substantially pure protein food ingredient. It is obtained by the thermal denaturization of collagen, the most common protein in the animal kingdom.
Properties of Gelatin:
Gelatin’s solubility in water and compatibility with aqueous solutions makes it very simple to alter gelatin hardness. Loss of water can result in losing gelation. In some instances, gelatin solubility is dependent on the charge of the protein molecule or the pH of the product. Gelatin’s adhesive properties depends on its temperature, and that the gelatin must not have gelled before surfaces are brought together. Gelatin’s gelling properties, most commonly its thermally reversible properties with water, produces the traditional Jell-O to which we are accustomed. Gelatin is used as a binder, stabilizer, thickener, or texturizer in foods.
The Effect of Gelatin in Common Food Products:
Some raw fruits contain proteases that break down the proteins in Jell-O, and therefore must be cooked before being added to gelatin solutions. Gelatin’s foaming capabilities, for example, are responsible for the texture of marshmallows. Frozen jelly with thaw upon disintegration and express water if the water is in excess; or, it will enable the excess water to freeze into small crystals if water content is at a minimum - this is critical for ice cream manufacturers to produce smooth, edible products. Gelatin’s emulsifying properties allow the creation of toffees and the ability for water and oil to combine in low fat margarine. It is used to stabilize dairy products and clarify fruit juices.
One method of testing for most effective use in food products is measuring its gel strength. Texture and strength of gel is critical for finding its effect on foods production, manufacture, and consumer satisfaction are dependent on gelatin’s properties. “Gel strength” is a quantity used to describe the qualitative nature of viscous gelatin. For the purposes of our experiment, the question we will be pursuing is how gel strength will indirectly and physically be measured by using the correlation between the amount of energy a projectile has as it is dropped into the gel and the resulting depth it has sunk in a predetermined amount of time. The research question we will be pursuing is what exactly that correlation will be. Our hypothesis is that a stronger gel will have more resistance and the projectile will only sink a small amount, whereas a weaker gel will cause the projectile to sink in a significant amount, possibly all the way through the gelatin. This correlation would be linear because of the direct proportion between energy, mass and height.
The way we will be calculating the amount of energy a projectile has as it is dropped into the gelatin is by using the relationship of mass, the gravitational constant, and the height from which the projectile is dropped to calculate the change in potential energy as the lead weight is dropped. The formula is E = mgh, where E is the change in potential energy as the lead weight is dropped, m is the mass of the weight, g is the gravitational constant 9.81 m/s, and h is the height from which the lead weight is dropped in meters.
Independent variables:
Energy of projectile (manipulated through changes to both mass and drop distance)
Dependent: Depth projectile sinks into the gelatin in 90 seconds
Control: Gelatin concentration, gelatin brand, pH of gelatin, temperature in which gels are formed and experiment is performed, electric charge of gelatin, time projectile is in the gel, containers in which the gel is set and the lead weights are dropped, the shape of the projectiles,
Materials:
Colorless gelatin
Water
Fishing weights of 15g, 20g, and 25g
Dental floss
Transparent cups at least 13.5 cm deep (glass preferred)
Ruler
Stopwatch
Hollow tube at least 48.8 cm in length
Stove/ Heating pan
Put 18 oz of water into a pot on the stove. Mix in 1 oz of gelatin and heat close to a boil. Stir periodically so the gelatin doesn't form a sticky layer on the bottom of the pot. A thin layer of minute white bubbles should form on the surface of the water. Once the white layer of bubbles is gone from the top of the water, turn off the stove. Let the mixture cool until it's safe to pour into the glasses. Fill the glasses close to the rim and all at equal heights. Depending on the size of the glasses, 18 oz of mixture might not fill very many. Twenty seven gelatin mixtures are needed for this experiment, so repeat the aforementioned steps the appropriate number of times.
Let the mixture cool into a stiff gelatin. It may take several hours. If the glass can be tilted parallel to the floor and the gel doesn't deform, then it has gelled completely and is ready to be used. For the most consistent results, let the gelatin set in a constant temperature so the density will be consistent across all trials.
Tie a length of floss to the loop on the fishing weight. Place the tube directly over the glass and as close as possible to the gel. Do not let the end of the tube touch the gel's surface as that would affect the weight's initial penetration. Hold the tube perpendicular to the rim of the glass. Another person may be needed to hold the tube while the weight is dropped.
Take the floss and hold the 15g weight 28.8 cm above the surface of the gel. If the tube is longer than 28.8 cm, the weight may need to dangle a into the tube before it is released. Hold the weight as still as possible; do not let it spin on the floss. Drop the weight while simultaneously starting the timer and remove the tube. Hold the ruler up against the glass and measure the initial penetration, making sure you are at eye-level with the weight for accuracy. Record the depth of the weight every 15 seconds for 2 minutes.
Repeat the instructions in the third paragraph three times for each weight. Drop the weights from 28.8cm, 38.8cm and 48.8cm, for a total of 27 drops (three weights, three drop heights, three trials for each drop height and weight).
RESULTS AND CALCULATIONS: Back to Top
With this range of data, error (and thus, error bars on the graph) is negligible.
Circumference of Weights:
Small: 3.8cm
Medium: 4.25cm
Large: 4.7cm
Cross-Sectional Area:
Small: 11.34cm²
Medium: 14.186cm²
Large: 17.35cm²
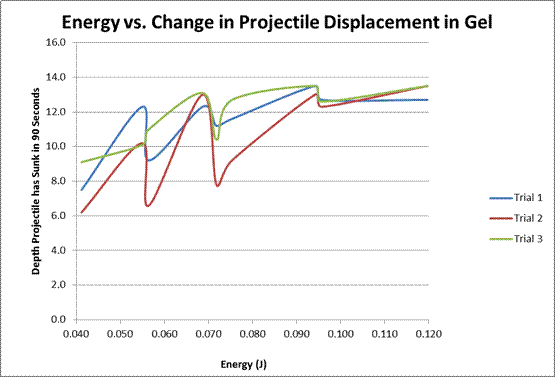
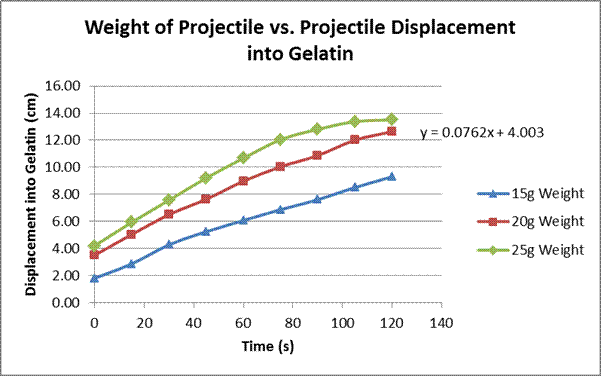
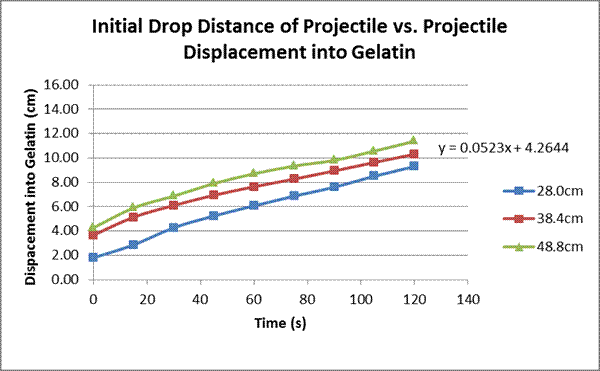
Our hypothesis was right: there was a linear correlation between the projectile’s energy (different through manipulations of both height and weight) and the displacement of the projectile into the gelatin after a short amount of time. Although the measurements were taken for two minutes, the first graph only shows results to a minute and a half. This is because after that some of the projectiles hit the bottom of the cup. This can be seen on the 25g weight line on the second graph, where it appears to flatten out at the end. This limitation is one of the contributing factors to the error in this experiment. One important thing to note is that on the first graph, although the basic structure is linear, the lines weave up and down almost like a polynomial. This is attested to the fact that the weight of the projectile has a greater effect on the displacement into the gelatin than the initial drop distance does. This is shown in the subsequent graphs; the slope on the graph with the weight manipulated is larger than the one on the graph with the drop distance manipulated. The greater effect the weight seems to have is most likely attributed to the fact that all three projectiles had a different cross-sectional area, ranging from 11.34cm² to 17.35 cm², a factor that was not contributed for in the calculations for the change in energy potential in our experiments. Thus, this is also another error in our experiment. Another, possibly more accurate, experiment that could be done is using projectiles with the same surface area and cross-sectional area, and using longer containers to hold the gelatin so the projectile cannot hit the bottom. A follow-up experiment to this one could be to look at different shapes of projectiles and how they effect entry: for example, pointed projectiles that could maybe cut through the gelatin easier, spherical projectiles with more even weight distribution, and/or with projectiles with flat faces to see its effect .
Bourne, Malcom. Food Texture and Viscosity: Concept and Measurement. New York: Academic Press, 1982. Print
Cole, CGB. Gelatin. Frederick J Francis, editor. Encyclopedia of Food Science and Technology, 2nd Edition. 4 Vols. New York: John Wiley & Sons, 2000. 1183-1188
Steffe, James. Rheological Methods in Food Process Engingeering. East Lansing, Michigan: Freeman Press, 1992. Print.
