Nick Sarka
Table of Contents:
Introduction| Top
What temperatures increase or decrease viscosity? Viscosity is the measurement of internal friction of a fluid. So for example regular maple syrup has a higher viscosity than water because the thickness of the syrup is greater than that of water. Iím curious to find out whether or not the temperature of oil can determine the viscosity of the liquid. I hypothesize that the temperature of oil will affect its viscosity in both negative and positive aspects; meaning that if the temperature of the oil is increased then the viscosity of the oil will therefore decrease. I believe that this may happen because of the increased energy in the liquid makes it more fluid as the particles of the oil are moving faster.
After collecting my data I will be using Google sheets to determine a line of best fit for the data.
Procedure + Design| Top
Design
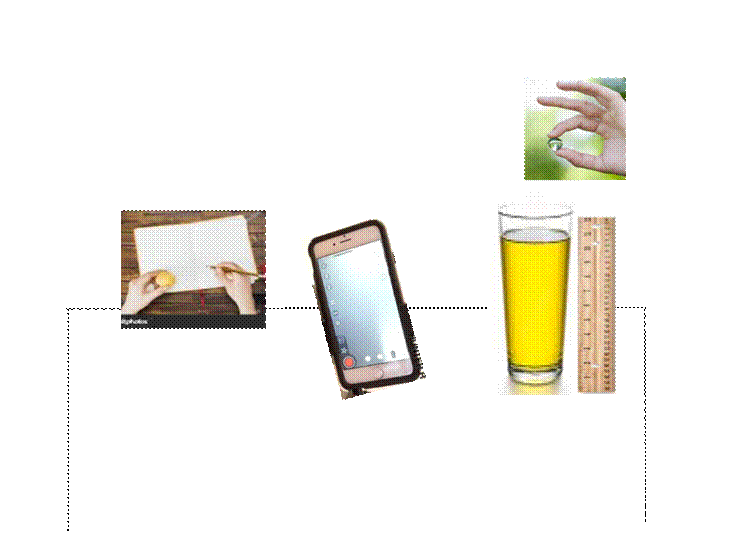
Materials
● Pencil
● Notebook
● 12 oz glass
● 8 oz of Olive Oil
● Phone camera
● Freezer or Fridge
● Microwave
● Marble
● Ruler
● Thermometer (Any kind will do)
● Spoon
Before I collected data, I set up my lab like so; I filled the glass with Olive Oil and set it on top of the counter. From there I grabbed a ruler to measure the top of the oil to the bottom of the oil so I could calculate the velocity of the marble. After measuring the height of the oil, I grabbed a thermometer and measured the temperature of the oil and recorded it in my notebook. Afterward, I put my phone in a place so that it has a level view of the glass and I started recording myself holding a marble at the top of the glass and then dropping it into the oil to the bottom. Once the marble hit the bottom of the glass I stopped the recording on my phone and viewed the video and recorded the times that it hit the top of the oil and when it hit the bottom of the glass. I repeated this with the same oil temperature 3 times and then proceeded to either put the glass of oil in the microwave and increase the temperature of the oil or put the glass of oil in the freezer and decrease the temperature.
Variables
In my experiment, I will be testing whether the temperature of oil affects its Viscosity. Every temperature will be measured with the same thermometer and the volume of the oil will be constant throughout the experiment. I will be able to obtain a fairly accurate time of the marble dropping because of the app that I will be using has the ability to record in HD (1080p) with 120 FPS (frames per second). The high FPS allows me to accurately measure when the marble touches the oil and when it touches the bottom.
Data Analysis| Top
Linear Regression
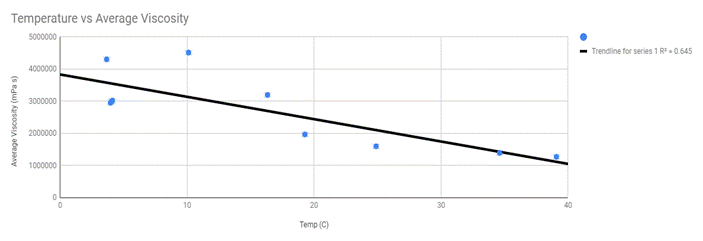

As you can see the linear regression line fits the averages very well while for the raw data the linear regression line is poorly connected to the data as its R-value = 0.2. I believe that representing my data with a linear regression line is too simple as has some limitations to the data that it can actually represent and the trend that Iím trying to show. If we were to continue following the trend line down, we would come across a point where the temperature of the oil would become too high and the viscosity of the oil would become unattainable as there wouldnít be any liquid. So the linear regression line is not the best fit for my data as it will not be able to show the actual trend of the decreasing viscosity that my data suggests and does not show the slowing of the rate of viscosity that I wish to represent. If I were to take more data points in between the gaps of temperatures, the data show that it is not a linear function.
Raw Data| Top
Calculating Viscosity
In order to find out the find out what the viscosity of the oil that Iím testing is, I need to set Stokes Equation equal to the Buoyant Force of the oil itself. After finding the buoyant force (by calculating the density of the oil times the volume times 9.81) of 6007.1535 N, I set it equal to Stokes Equation and solved for the viscosity using Algebra.
Power Series Regression
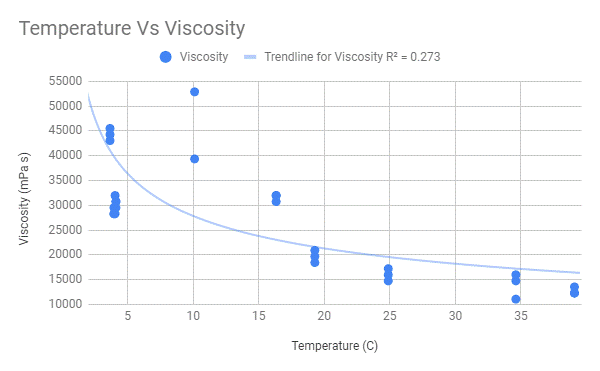
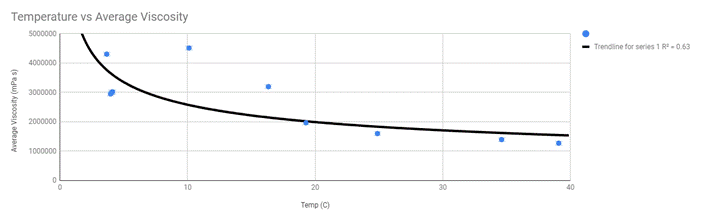
A power series curve I believe accurately depicts the trend in my data that I wish to present as my results. Also, another piece of evidence to support my choice would be the closeness in R-values as they are significantly closer than those of the linear regression lines. Also, the power series regression curves show the story of the viscosity of oil that my data actually shows, as the temperature increases the difference in viscosity between temperatures decreases. If I were to take more data points in between the gaps of temperatures, the data show that it is a power series function.
Conclusion/Evaluation| Top
As I hypothesized earlier, the viscosity of oil was affected by the temperature of the oil itself in negative and positive ways.
It is also evident that the power series function is the curve that best fits the data that I have collected as the correlation coefficient is much higher than those of the linear regression lines.
Thereís plenty of errors and limitations that occurred throughout the experimental process. For example, this experiment would have benefited greatly from more data so that my claims of finding the best fit line is supported more clearly and my conclusions can be ever more confident. Because of the time constraint that I was in, the amount of trials and variations that I could conduct were limited to the ones that I display in my tables.
Another error that came in my experiment is the changing of temperatures. As I recorded and calculated the data needed for the viscosity of the oil, there was a considerable amount of time between trials so the temperature of the oil may have fluctuated during that time. This, therefore, draws some validity away from my results as the viscosities may not be truly what they are at the temperature recorded.
Overall, Iím curious to figure out if the viscosity of oil could ever equal the viscosity of water. I doubt that ever could be true because of the inherent chemical compound difference. Surprisingly, I did not need to look up anything other than the density of Olive Oil so that I could calculate the Buoyant force.
Bibliography| Top
Elert, Glenn.
ďDensity of Cooking Oil.Ē E-World,
2010, hypertextbook.com/facts/2000/IngaDorfman.shtml.
Links for More fun| Top
Video showing how to test for Viscosity: https://youtu.be/2Gdxu4XcsbY
Website giving tips for measuring viscosity in oil: https://www.machinerylubrication.com/Read/30428/testing-oil-viscosity
Physics HyperTextbook(Viscosity): https://physics.info/viscosity/resources.shtml
Really Cool Laminar Flow Water Faucet: https://www.youtube.com/watch?v=990jkBGHaVk
Video Explaing Viscosity and showing examples of different viscosities: https://www.youtube.com/watch?v=f6spBkVeQ4w