Temperature Effects on the Elasticity of Rubber Bands
By Anna Black, Ellen McKean, and Madison Richardson
Table of Contents
Background Information | Purpose | Hypothesis | Method | Diagrams and Pictures | Data and Graph | Conclusion | Sources | Go Up
Background Information
First used by Caribbean locals, Christopher Columbus was the first to bring
rubber to Europe. Later in 1839, Charles Goodyear discovers heating the heating
process of Vulcanization, which stabilized and strengthened rubber’s
properties. The addition of sulfur induces vulcanization, which connects rubber
molecules into a chain, giving rubber bands their elasticity, strength, and
making it immune to solvents and moderate heat and cold. The method of
vulcanization allowed for the more widespread use of rubber. Synthetic rubber
is formed by adding color, sulfur, and other additives to make the material
last longer. (Rubber World Vol. 214) To make rubber bands, the
previous ingredients are mixed in a container that is heated to 250 degrees
Fahrenheit until it is a ball of dough. Then it is rolled into sheets, cut into
strips, then molded into the shape of a tube, and later is heated, rinsed,
cooled and cut (www.rubber-band.com).
Rubber has unique viscoelastic qualities, meaning it has the elastic properties
of a metal spring, but also has the energy absorbing characteristics of a
viscous liquid. These properties allow rubber to maintain its original shape
after deformation (Rubber World Vol. 210). Also, if rubber is stretched it will
actually shrink when heated, but is also less resilient when cooled, because
the strands become less mobile. It shrinks because as the molecules in the
rubber become heated, they also become less aligned and contract, causing the
rubber band itself to shrink. This simple experiment can be performed by using
weights to stretch the rubber band 2-4 times its original length, then heating
it with a hair dryer. The rubber band will shrink, but then will return to its
original length after cooling (http://www.newton.dep.anl.gov).
Purpose
The purpose of this investigation is to determine the correlation between
the stretch distance of a rubber band and the temperature to which it is
subjected.
Hypothesis
Heat will affect the stretch distance of rubber bands because it alters
the bonds between the rubber molecules. When the heat increases, the bonds
between molecules will weaken causing the rubber band to stretch father. When
the temperature decreases, the molecular bonds strengthen causing the rubber
band to stiffen up and not stretch as far. The time that the rubber bands are
subjected to the varying temperatures, brand of rubber band, and the force
applied will remain constant while the temperature changes.
For this experiment there is a separate set up for both warmer and cooler temperatures. The cold set up requires a small cooler, which we filled 4.77cm full of ice. We then added 1 tablespoon of salt to the ice. A rack on which the rubber bands were placed was suspended over the ice. For the warm temperatures, a conventional oven was set at a temperature of 350° Fahrenheit. In the oven, a non stick, non metal cooking sheet was placed to separate the rubber bands from the hot metal surface of the oven. The rubber bands were then placed in their respective set ups, and removed when they reached the desired temperature. Temperature was measured by an electronic thermometer. Four cold temperatures were taken, beginning at 65 F and decreasing by 5°F. The warm temperatures began at 80 °F and varied by 10°F. There were a total of 5 warm temperatures. The was also one room temperature test at 70°F. For each temperature, we collected data for two colored rubber bands, and two natural rubber bands. When the rubber bands were removed from their apparatuses, a measurement was taken pre-stretching. The rubber bands were suspended from a secured pencil, and 400g of weight were hung from the pencil. The length of the stretching pencil was taken, and the original length was subtracted from it to find the stretch distance. All data was converted to Celsius at the end of the experiment.
Cold Apparatus
 |
|
 |
Warm Apparatus
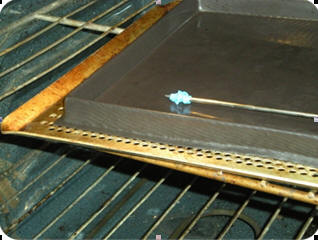 |
 |
 |
Heat Effects on Regular Rubber Bands
|
Temperature (°C) |
Length after Heating (cm) |
Length after Stretching (cm) |
Stretch Distance (cm) |
|
|
7.5 |
10.3 |
2.8 |
|
|
0.00 |
7.5 |
10.2 |
2.7 |
|
|
7.20 |
7.6 |
10.6 |
3.0 |
|
|
7.20 |
7.5 |
10.7 |
3.2 |
|
|
16.10 |
7.8 |
11.0 |
3.2 |
|
|
16.10 |
7.8 |
11.0 |
3.2 |
|
|
18.90 |
7.5 |
9.5 |
2.0 |
|
|
18.90 |
7.7 |
10.9 |
3.0 |
|
|
21.10 |
7.8 |
13.2 |
5.4 |
|
|
21.10 |
7.6 |
11.0 |
3.4 |
|
|
28.20 |
7.5 |
11.2 |
3.7 |
|
|
28.20 |
7.8 |
11.5 |
3.7 |
|
|
33.30 |
7.8 |
13.3 |
5.5 |
|
|
33.30 |
7.7 |
11.6 |
3.9 |
|
|
38.75 |
7.9 |
10.9 |
3.0 |
|
|
38.75 |
7.6 |
9.4 |
1.8 |
|
|
43.89 |
7.8 |
10.1 |
2.3 |
|
|
43.89 |
7.6 |
9.9 |
2.6 |
|
|
47.78 |
7.7 |
10.3 |
2.6 |
|
|
47.78 |
7.8 |
10.4 |
2.6 |
Heat Effects on Colored Rubber Bands
|
Temperature (°C) |
Length after Heating (cm) |
Length after Stretching (cm) |
Stretch Distance (cm) |
|
|
7.5 |
13.3 |
5.8 |
|
|
0.00 |
8.4 |
15.2 |
6.8 |
|
|
7.20 |
7.6 |
13.9 |
6.3 |
|
|
7.20 |
8.0 |
14.1 |
6.1 |
|
|
16.10 |
8.1 |
14.7 |
6.6 |
|
|
16.10 |
7.6 |
14.5 |
6.9 |
|
|
18.90 |
8.9 |
20.1 |
11.2 |
|
|
18.90 |
8.2 |
14.0 |
5.8 |
|
|
21.10 |
8.0 |
14.6 |
6.6 |
|
|
21.10 |
8.0 |
13.4 |
5.4 |
|
|
28.20 |
8.0 |
14.8 |
8.8 |
|
|
28.20 |
8.0 |
15.8 |
7.8 |
|
|
33.30 |
8.5 |
15.4 |
6.9 |
|
|
33.30 |
8.4 |
17.4 |
9.0 |
|
|
38.75 |
8.3 |
14.3 |
6.0 |
|
|
38.75 |
8.0 |
16.1 |
8.1 |
|
|
43.89 |
8.3 |
16.4 |
8.1 |
|
|
43.89 |
8.3 |
15.4 |
7.1 |
|
|
47.78 |
7.2 |
13.2 |
6.0 |
|
|
47.78 |
7.0 |
12.6 |
5.6 |

The results that we got from our experiment were very sporadic, not linear or consistently curving. The colored rubber bands in particular had the largest range between extreme stretch distances (5.4 cm – 11.2 cm; range of 5.8 cm). This may have been caused by an ingredient in the dye, such as water, that altered the rubber’s response to vulcanization. We can assume this because of the consistency of the regular rubber bands whose extreme values (1.8 cm and 5.5cm) where only separated by 3.7 cm. Although the rubber bands used had the same thickness, the colored rubber bands were less resistant to the weight. The data seems to suggest that there is no significant variance between the cooled, room temperature, and heated rubber bands. This simply reiterates the concept of vulcanization. The manufactures of these rubber bands used vulcanization to prevent the effect of temperature on their product. Our experiment seems to prove the effectiveness of vulcanization in stabilizing the molecular structure of the rubber bands.
There are several errors that may have occurred. First, the thermometer used was a cooking thermometer, so it had difficulty reading the extremely cold temperatures. It also didn’t show the temperature changing by degree, but rather would show one temperature and then jump sometimes ten degrees to the next temperature. This made data very difficult to collect precisely and hard to duplicate with another trial. The second error is that we were unable to keep the rubber at the measured temperature as we measured its length and stretch distance. The band’s temperature changed immediately as soon as we removed it from the oven or the cooling box. Unless we preformed the experiment in a room with the same temperature that we wanted our rubber bands to be at, there isn’t a way to eliminate this error. Our final error is that as we put the rubber bands on the thermometer, we could not keep the tension the same. Because of that, some rubber bands may have stretched more than others before their heating/cooling process, making them stretch more with the weight. Again, this is a difficult, if not impossible error to overcome. We were as careful as possible to keep the rubber bands loose.
http://www.rubberband.com/. Aristotle, n.d. Web. 10 Oct. 2010.
This is the Alliance Rubber Company's website. This site was useful for background information concerning the history of commercial rubber use and manufacturing process of rubber bands.
Calder , Vince. "Rubber Bands and Elasticity." http://www.newton.dep.anl.gov.
N.p., n.d. Web.
10 Oct. 2010. <http://www.newton.dep.anl.gov/askasci/eng99/eng99163.htm>;.
On this site we found a similar experimental method, that helped us to both design our own lab setup, and to avoid previously made errors.
Columbia Electronic Encyclopedia, 6th Edition; 7/1/2010, p1-1, 1p
Rubber World; May94, Vol. 210 Issue 2, p20, 3p, 1 Black and White Photograph, 1 Graph (Rubber World Vol. 210)
Rubber World; Apr96, Vol. 214 Issue 1, p18, 3p, 1 Black and White Photograph, 1 Diagram, 1 Graph (Rubber World Vol. 214)
The above three sources provided information about the process of vulcanization, and the chemical composition of rubber bands.