Researching the Ultimate Effiecency of a Fixed Volume Force Gauge:
Christian Bennett
Erich Helmreich
Alec Rietman
Background | Problem | Hypothesis | Materials | Procedure | Data | Conclusion | Related Websites | Go Up
Background: .:. Go Up .:.
Combustion has been used since the dawn of time in matters of practicality and for entertainment. The origin of this research resides with a popular backyard project, the potato cannon. A basic series of plastic plumbing parts, some cement and a spark, that when using a propellant, will launch a potato hundreds of yards. The principal is that a spark will ignite a largely propane based aerosol, that expands violently to propel the projectile. The force of the explosion is very unpredictable, due to the metering of propellant, oxygen saturation, and a myriad of factors that hinder reliable combustion, often forming as a side effect of the combustion. Most of these factors can be controlled in the right environment. In a quest to maximize the force of the projectile, thus the force of the explosion, the key factor comes to propane metering. (the propellant of choice)
All fuel air explosions, as implied by the name, require a fuel and an oxidizer. Propane and oxygen in this case. In a space with a limited supply of oxygen, there must be a precise amount of propane (or any fuel) in order to achieve the largest and most efficient combustion process. Meaning that there should be exactly enough oxygen to completely combust the propane. Because chemistry dictates that the number of moles of propane and oxygen (“air”) must equal the number of moles of carbon dioxide and water plus heat.
The 'golden ratio' is called The Stoichiometric Air-fuel Ratio (Bennett 2002). The Stoichiometric Air-fuel ratio is obtained when the amount of reactants, propane and oxygen, equal one another and no residue or reactant is left over after the combustion. This ratio will be considered after the initial graphical representation of force as a function of the volume of fuel. In theory, the stoichometric ratio should show a parabola function of data points of which a function representing the variation in force compared to the volume of fuel.
Every object that uses combustion is rooted in a principal nearly identical to this. In order to maximize efficiency, huge, ridiculously large sums of money, time and effort are spent researching and developing new ways to increase efficiency. From heating to transportation, research into efficiency of chemical energy is a crutial step to a more developed future utilizing the resources that we have.
Statement of the Problem: .:. Go Up .:.
The purpose of this investigation is to determine what volume of propane fuel will result in the largest force of combustion within a confined space. (Force gauge)
Hypothesis: .:. Go Up .:.
If a number of specific volumes of propane are detonated inside a controlled combustion chamber; then the resulting force of the explosion, measured by the velocity of a known projectile which can be used to plot point on a graph to determine an optimal fuel density because force us equal to mass times acceleration and the efficiency of an explosion dictates the force, due to a limited supply of O2; the resulting force will increase as fuel increase.
Materials: .:. Go Up .:.
Force Gauge (Potato Canon) (And all accessories needed to use)
Water Balloons
Propane
Vernier’s Logger Pro 3.6.0 Software
60 Frame per Second Camera
Wooden Dowel (1 ¼”*8’)
Foam Encasement for projectile
Electronic scale
Meter stick
Ti-89
Procedure: .:. Go Up .:.
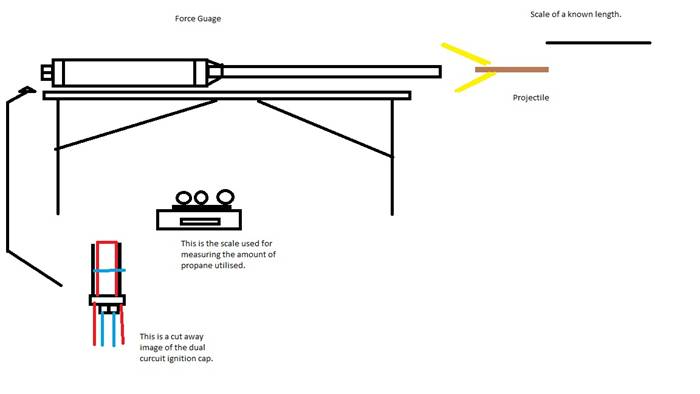
To repeat this experiment, one must first obtain all of the materials listed above. It is easiest to have multiple people working on this lab at the same time as you can accomplish more in less time. You must set up the scale where it will not be disturbed by anything as it can be a very delicate piece of machinery. You will be using it to measure the amount of propane that eventually goes into the canon, which needs to be accurate. For every shot, select three balloons from the selection you have acquired, and weigh them with the scale. Record the weight as you will need it in your calculations. Next you must fill the balloons with propane. We chose to use a nozzle attached directly onto the propane canister. After you have filled the balloons with propane you need to weigh them. What works best to weigh them on a small scale is to get a holding container and zero the scale with it on the scale. Then add the balloons to the box and read the weight. Record this number as well, as it is also needed later in the experiment. Next we must weigh the projectile which is a wooden rod with a sabot around it. Record this number with intentions of using it later. Next load the cannon with the projectile. We must now put the propellant into the chamber to be fired. Our ingenious way of doing this was to tape the balloons to a long strip of wire that wouldn’t burn through if a current ran through it, for more information please see the diagram located in this report. It would just pop the balloons and not ignite the propane. Connect a filament to two leads attached to the second set of wires. This filament will burn through and ignite the combustion chamber Afterwards the cannon is ready to fire. Press the propane release switch, and then wait ten seconds. After that, press the ignition switch and voila you should have combustion. All you have to do is get your camera to record the shot being fired with an object in the background that you know the length of. You will use this video in the software program Logger Pro to get the velocity of the projectile. Subsequently you must do this over and over again until you attain numerous data points. From there it is a matter of taking velocity and time, from Logger Pro, and converting it to acceleration. With that you multiple the acceleration by the mass of the projectile, which you wrote down earlier, and the answer is the force exerted. For each mass of propane, graph the resulting force (x=mass of propane, y=force) and then find the parabolic equation for your graph. Using a TI-89 calculator plug your equation in, and select 2nd and TRACE. From there select maximum, and click in the left bound and the right bound. The number that is displayed is the ideal amount of propane in the chamber per shot to attain maximum efficiency.
Data .:. Go Up .:.

Conclusion and Analysis .:. Go Up .:.
Our hypothesis, that the force of the combustion would increase as mass of fuel increased, was wrong. The results indicate that the ideal mass of propane in this combustion chamber would be .7924 grams of propane.
Error Analysis .:. Go Up .:.
The source for error in our experiment were minimized as often as humanly possible. Following the procedures step by step allows us to determine error throughout the experiment. The first possible source of error is in calculating the mass of propane in the balloons. Although theoretically using the correct way to discover this, zeroing a container and than weighing the balloons in it, the way the balloons were placed in it were not constant. Error could also be seen in possible leakage of propane from balloons, or from the back of the cannon. Another source of error could be the location of the filament in the combustion chamber. The filament was placed slightly in the middle of the chamber and thus with ignition directed force away from the location of burn meaning that force was exerted backwards, towards the cap on the back of the force gauge. This would manifest error in actual force exerted. This doesn’t mean our calculations for force are wrong it just means that if someone else was to go about this experiment and use a different method to measure force, perhaps the numbers they would get might be different if they measured exact force like from a ballistic pendulum or something like that. Overall, other sources of error could include inaccurately creating points in Logger Pro, or typing up the wrong number, or reading the scale incorrectly. There isn’t cause for alarm of error on these last three mentioned, but they should always be noted for future experiments. Finding a way to control human error would be great.
Related Websites .:. Go Up .:.
http://www.process-heating.com/Articles/Energy_Notes/abdb99d56e268010VgnVCM100000f932a8c0____---Description of the Propane-Air Ratio
http://www.springerlink.com/content/u47752630um31118/---Perfecting an Air-Propane Mixture
http://www.slideshare.net/marcusforpresident2012/green-engines-development-using-compressed-natural-gas-as-an-alternative-fuel---Propane/Natural Gas as a alternative fuel
http://www.amtonline.com/publication/article.jsp?pubId=1&id=1171---Air-Fuel Ratios and solving stochiometry
http://www.engineeringtoolbox.com/stoichiometric-combustion-d_399.html---Solving Air-fuel ratios
http://tiny.cc/7a1my --- Entertaining Website