Great Balls of Fire

An investigation of balloons, fire, explosions, light, and testosterone-fueled teenagers.
Return to Physics Website
Great Balls of Fire

An investigation of balloons, fire, explosions, light, and testosterone-fueled teenagers.
Background Information
Information that only mildly pertains to the subject.
Lumens are the SI unit of measure for luminous flux, the degrees of light perceivable by the human eye. Brightness is considered the light emitted from a surface per unit of area. Luminance (lumens) also includes direction as a factor of the intensity of light.
Propane is a three-carbon alkane derived from petroleum or natural gas; it is often sold as liquid petroleum gas (LPG or LP-Gas). Propane undergoes combustion in the presence of excess oxygen, producing only water and carbon dioxide: C3H8 + 5O2 ? 3CO2 + 4H2O + heat. It can also produce water and carbon monoxide from combustion: 2C3H8 + 7O2 ? 6CO + 8H2O + heat. As both methods of combustion represent, heat is a significant part of the result. As such, propane is often combusted to produce heat as a form of energy. Logically, propane could be placed in the correct conditions to produce extreme heat, such as a flame or explosion.
Return to top.
Statement of Intent
Most likely an intentional statement.
The purpose of this investigation is to determine the relationship between the amount of propane inserted into a balloon and the luminance produced by the combustion of said balloon.
Return to top.
Review of Literature
More like Wall of Text, amirite?
Due to its potential as an alternative fuel source, as well as its application in heating mechanisms, a great deal of research is conducted on the combustion of propane. According to the research of E. E. Lin and Z. V. Tanakov (Russian Federal Nuclear Center) regarding the combustion of hydrocarbons in an open cloud environment, “... mixture ignition occurs in a set of the local centers on the [propane] cloud surface. During the stretch of time near 0.1 s these centers flow together in uniform burning spot with the characteristic size of 1 m. Subsequently, this spot increases and propagates on the cloud surface either as continuous field of luminescence on the all cloud height...” (2006). This research provides a basis for understanding the combustion of propane when it is released into an open environment. Research into plasma-enhanced propane combustion, performed by L. A. Rosocha, D. M. Coates, D. Platts, and S. Stange, stated a relationship between reaction rate and temperature, “Burning then continues by the propagation of the reactive species generated by the heat of the reaction itself. Thus, the overall combustion reaction rate is usually determined by the efficiency of generation of the new reactive species in the spreading flame front. The faster the reaction rate, the higher the temperature of the combustion process, with detonations producing the highest temperatures and fastest pressure rises. The efficiency of the combustion process is largely determined by usual thermodynamic considerations, namely, the higher the temperature, the more thorough and efficient the combustion process becomes” (2004). G. I. Kozlov's investigation of the propagation of pyrolysis and combustion waves in propane suggested that an increase in pressure of the propane gas would produce an accelerated combustion, “when the propane pressure was increased from 25 to 100 Torr and above, the propagation velocity of the front increased appreciably to 1.3 m/s near the focusing region. However, the front then exhibited unusual behavior, where it propagated more slowly, stopped, and even withdrew slightly back to the focus. After a few frames, the process appeared to recover and the pyrolysis wavefront continued to propagate along the beam” (1998). From these reports, it would seem reasonable to state that an increase in pressure, which would increase the velocity of the combustion, which would, in turn, increase the temperature attained by the combustion process. The 42nd volume of Combustion, Explosion, and Shock Waves, explains the manner in which a thermal explosion would occur, “In the case of a nonuniform distribution of the induction time of the reaction, a local explosion of a thermal nature occurs, resulting in detonation development exclusively in the direction of increasing values of t [local-explosion delay]” (2003). V. A. Bunav and V. S. Babkin's research of propane-air interaction in a low-temperature environment explains the relationship between the temperature of the surrounding environment with the temperature at which a propane substance combusts, “Indeed, from a comparison of the temperatures at the beginning of the formation of the main products H2O, CO, and CO2 under flame conditions with the self-ignition temperature of propane–air mixtures at atmospheric pressure (750–900 K [10]), it can be concluded that the reason for the significant difference is related to the diffusion flows of the lightest products of the chemical transformation to the heating zone.” (2006). The difference referenced is in regards to the self-ignition temperature of a propane-air substance, which can change under flame conditions.
Return to top.
Hypothesis
Prior to the project, we hypothesized that this would be our hypothesis.
Due to the concept that an increased pressure would produce a greater change in temperature (as suggested under the Review of Literature), we believe that the light produced by the combustion of a propane balloon would increase as the amount of propane ignited increases. The greater change in temperature suggests a greater amount of energy produced which could produce more luminescence (as measured in lumen seconds). The amount of propane will be measured via the volume of the propane-filled balloon.
Return to top.
Overview
If you haven't figured it outyet.
We will fill balloons with varying amounts of propane and take multiple measurements of different circumferences to derive an average circumference and from that derive the volume of the balloon. We will then fix the balloon in a dark place in front of a light meter, which is being recorded and had been calibrated to the existing light. We will then ignite the propane balloons with a propane torch fixed to a pole and record our results.
Return to top.
Materials
I would recommend you keep these in stead supply if you like fireballs.
Diagram
Ignore the crappy colors, paint doesn't like me...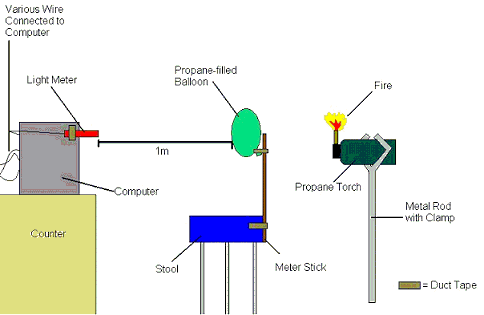
Diagram A-1
Procedures
Loads of fun ensue
To begin, gather the required materials listed above, and re-create the set-up shown in diagram A-1. Duct tape a meter stick to a chair to create a solid base for the balloon. Attach the light meter to the computer, making sure it is one meter away from the base and on the same plane. Make the room as dark as possible. Open Logger Pro and follow the presented instructions to calibrate the light present in the room to zero. Afterwards turn on the lights to accommodate further set-up. To create the torch, fix the propane torch to the end of a rod of roughly 1 meter with a metal clamp. Make sure the rod-clamp structure is capable of supporting the weight of the propane torch. On site, make sure to operate in an environment clear of obstruction (especially flammable obstructions) and always have a fire blanket and fire extinguisher on hand during all experimentation. To prepare the propane balloons, attach the balloon to the nozzle of the propane tank (using duct tape to thicken nozzle where necessary), and fill with propane to the estimated size desired. Proceed to remove the balloon from the tank, making sure the end is secure, and tie it off tightly to insure full retention of gas. Then, use a string and meter stick to take 2 representative measurements of the circumference and record the average. Then fasten the balloon to the vertical base using duct tape, and prepare for ignition. Turn off the lights, ignite the propane torch using matches, begin recording data points with Logger Pro, and thrust the torch into the balloon to ignite the propane. Stop recording and save results. After repeating with many balloons of varying dimensions, calculate the respective integrals of the resulting graphs to determine total lux*s (lumen seconds) produced with each explosion. This can be done through a function built-in to Logger Pro. Graph results for analysis, calculate correlation coefficients and investigate possible regression lines.
Return to top.
Results
Sample Integral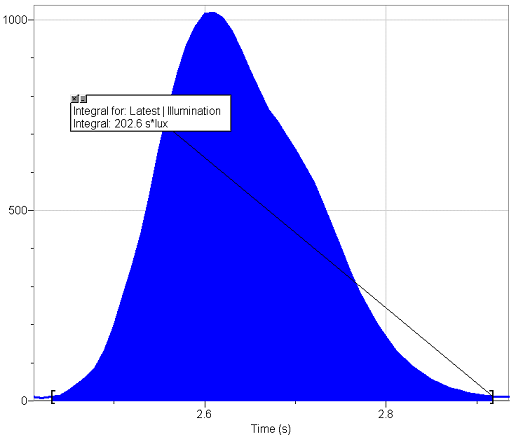
Data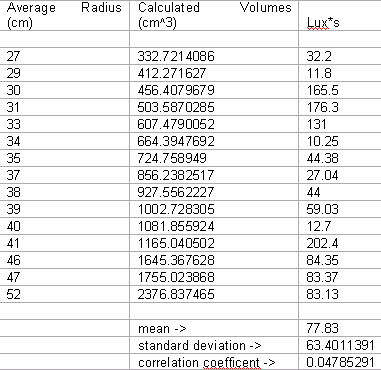
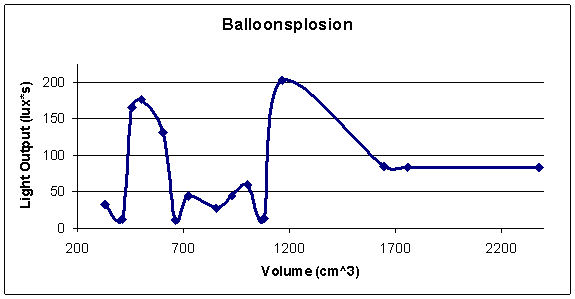
Return to top.
Conclusion
All that fantastic data, in paragraph form
The data does not support our hypothesis. The results of the experiment show little chance of correlation in our data, with a correlation coefficient of .048. Additionally, the graph does not seem to show a direct correlation, and no line of regression adequately described the data. It is possible that there was an ideal propane to gas ratio existing at around 450-600 cubic centimeters, as 3 data points would suggest, however too little concentration of data exists within that range to make an adequate assessment. The same is true for the low points visible between the 700-1100 cubic centimeters. Volumes from this range consistently produced a smaller amount of lux*s then nearly all other volumes (except for 400 cubic centimeters).
There were also several errors which may in part account for the seemingly sporadic results. Firstly, the balloons would exponentially increase in pressure as they increased in size, due to their elasticity. Secondly, because the balloons were not perfectly spherical, any measurement of volume would prove at least partially inaccurate. Thirdly, perfect darkness could not be achieved, and although the light meter had been calibrated accordingly, this may have still lead to errors in ratios. Fourthly, and most importantly, the light meter could only assess the light directly in front of it, and as our video analysis suggests, the much of the explosion tended to travel away from the light meter, usually in the direction of the torch. We believe this to have been caused by propane rushing out of the newly punctured hole due to the pressure of the propane. This notion is supported by the fact that the rush of propane would often extinguish the propane torch, sometimes not igniting the propane itself. On occasion the shape of the explosion would be extremely distorted, which would not allow the light meter to record the entirety of the explosion. Furthermore, it was difficult to ignite each balloon in a uniform manner. Lastly, human error in measurements is inevitably present.
The results of this experiment do, however, warrant further testing, especially to determine whether the apparent trend in the 450-600 and the 700-1100 cubic centimeter ranges would consistently provide similar results. An internalized ignition system would allow for a more uniform explosion and thereby better results. Also, an adequately calibrated scale would allow for testing the mass of propane instead of the volume, which would then avoid the issue with pressure, or perhaps incorporating a penetrometer to help account for pressure. Additionally, containers that created less pressure than balloons would also help to reduce the erratic results.
Return to top.
Bibliography
Bunev, V. A. and Babkin, V. S. Chemical Reactions in the Low-Temperature Zone of a Laminar Rich Propane-Air Flame. Institute of Technical Physics, Russian Federal Nuclear Center. 2006.
Coates, D. M., Platts, D., Rosocha, L. A., and Stange, S. Plasma-enhanced combustion of propane using a silent discharge. Los Alamos, New Mexico: Los Alamos National Laboratory. 2004.
Kozlov, G. I. Investigation of the laws governing the propagating of pyrolysis and combustion waves along a laser beam in propane. Moscow: Institute of Problems in Mechanics, Russian Academy of Sciences. 1997.
Lin, E. E. and Tanakov, Z. V. Features of Transitional Regimes for Hydrocarbon Combustosed Volumes and in Opened Clouds. Russian Federal Nuclear Center 2006.
Tarzhanov, V. I. Detonation of Propane-Air Mixtures under Injection of Hot Detonation Products. Institute of Technical Physics, Russian Federal Nuclear Center. 2006.
Return to top.
Related Links
Actual Documents
Research Project Write-Up
Data Files: Excel | Text -
Tab Delimited
Outside Information
Definition of Luminosity
Luminosity is a relatively significant aspect of our experiment, therein the definition might be useful.
Properties of Propane
These properties may partially explain the specific explosion pattern observable during our experiment
Black-Body Radiation
Black-body radiation is released by the combustion of propane in this instance, evident by the heat felt in the direct path of the explosion.
Use and Function of Light Meter
An explanation of light meters and their application to our experiment
Gas Laws
An explanation of gas laws which are hopefully applicable to our research project