Methanol Cannon
Researched by J Michael
Harrison, Jonathan White,
and Carl Glissmeyer
Table of Contents
Background Information
:: Review of Literature :: Question
:: Hypothesis :: Procedure/Method
Discussion :: Conclusion
:: Related Sites :: Bibliography
:: Return to Research Page
Background Information .:.
Top
The first cannons were created by the Chinese using simple bamboo casing and gunpowder. Europeans later refined this technology and used metal to envelop the cannon cavity, thus making them far more effect in durability, range, and intensity. The basic physics of a cannon is that the ignition source (flame, spark, etc.) ignites the fuel (gunpowder, methanol, etc.), thus causing the gas(es) inside the cannon chamber to combust and force the projectile out. For our experiment we plan to use methanol. This chemical compound is flammable in liquid form, but becomes extremely explosive as a gas. It has been used as a fuel in a wide variety of industrial processes, and it can be used as an alcohol fuel in different types of gasolines. Aside from the chemical components of a cannon, there is also the motion of the projectile itself. Principles of projectile motion were first described accurately by Galileo, who showed that motion could be analyzed by separating horizontal and vertical components. These realizations helped physicist arrive to the many formulas today that involve time, velocity, distance, and acceleration. These formulas will allow us to measure certain variables and apply them to our question.
Review of literature .:.
Top
In building a cannon which utilizes the combustion of methanol, there are many variables which can affect the initial velocity of the projectile. We intend to focus on the amount and concentration of methanol in the barrel, and as a result, there are many factors that will be kept constant. These include the mass and shape of the projectile (in this case a small tennis ball), the diameter, length, and overall composition of the barrel, and the ignition apparatus. Methanol is a colorless, volatile liquid and can pose a dangerous fire risk (Condensed Chemical Dictionary, Twelfth Edition). Its molecular structure, CH3OH, is not unlike most alcohols (Trefil, 2001). It readily and violently combusts when exposed to an ignition source (2001), and has a flash point of 14o C (Oxford University Chemistry Laboratory, 2004). Also supported by the Oxford Chemistry Lab is the notion that methanol is more potent as a vapor, which can be useful in our experiment. This information will be essential in successfully igniting the ethanol in the cannon. Measuring the initial velocity of the projectile (tennis ball) presents a logistical problem. It will be difficult to fire the cannon horizontally, as we hope it will launch the projectile at a substantially fast velocity, and we will have a limited area to experiment on. This problem can be solved by firing the cannon at a constant angle, as we can find the initial velocity by relating the angle of firing with the (negative) acceleration of gravity (Giancoli, 1998).
Description of the question .:.
Top
The question we are trying to explore is: “what is the relationship between the amount of methanol used to propel a tennis ball and the initial velocity it leaves the cannon with? Is it linear, logarithmic, exponential, etc.?” Therefore, in this exploration the amount of methanol gas used to project the tennis ball will be manipulated in order to determine the maximum initial velocity. Thus, the independent variable in this project will be the amount of methanol used to fire the ball, and the dependant variable will be the speed at which it leaves the cannon (initial velocity).
Hypothesis .:. Top
If the amount of methanol gas used (independent variable) to fuel the projection of a ball through a cannon is increased, then the initial velocity (dependant variable) will gradually increase in a logarithmic pattern, reaching an assumed limit.
Method/Procedures .:.
Top
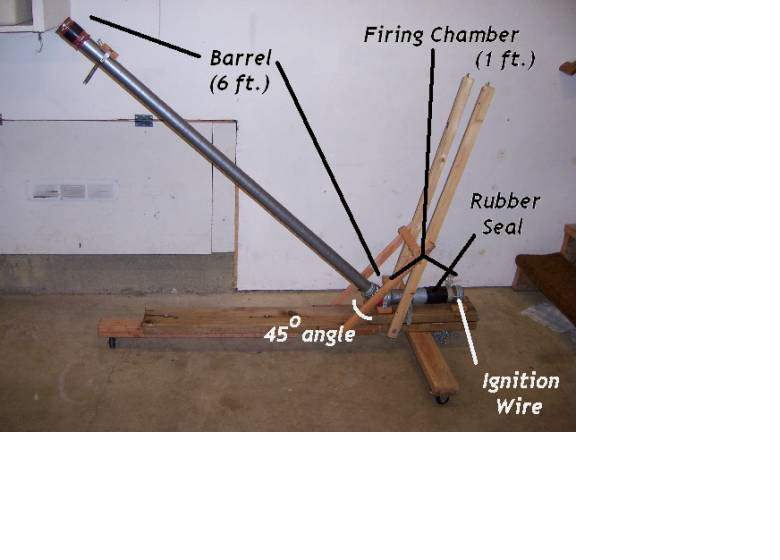
1. Place the projectile (the ball) in the very bottom of the barrel, adjacent to the fuel chamber, guaranteeing a tight fit between the projectile and the diameter of the barrel.
2. Using a spray bottle, “mist” the desired amount of methanol alcohol into the fuel chamber. Seal the injection point with a rubber sleeve.
3. Shake the methanol which has collected at the bottom of the fuel chamber by raising and lowering the trajectory angle of the cannon.
4. Power the Tesla Coil, and place a charge on the wire connecting to the fuel chamber; the alcohol within the chamber will ignite and eject the ball..
5. Mark the location where the ball first struck the ground, and measure the distance from the base of the barrel to the placement of the ball.
6. Conduct at least 15 tests, using 4 different amounts of methanol alcohol to project the ball, and record the distance traveled by the ball. Amounts used should be 10mL, 20mL, 30mL, and 40mL.
7. Using the data collected from the experiment, analyze the data through graphing it with an x-y scatter plot to determine the ratio between the fuel amount and initial velocity.
Picture :
Discussion .:. Top
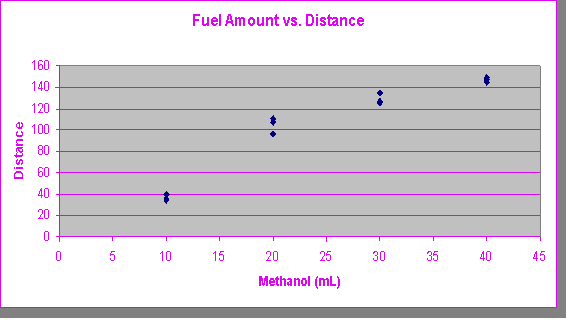
The data collected was initially sorted by the amount of fuel used to fire the projectile, and the distance the projectile covered after leaving the barrel. The data collected is shown here. It should be noted that three anomalous data points were eliminated: (10 mL fuel, 3.5 m traveled), (20 mL, 56.0 m), and (40 mL, 47.5 m).
*** Link To Raw Data ***
Using this information (the distance that the ball traveled), two elements were derived. First, the amount of fuel correlated with the distance traveled showed the relatively logarithmic relationship between the two variables. Graphically, this is shown with an x-y scatter plot (next page). The distance rises dramatically only to level off as methanol levels increased.
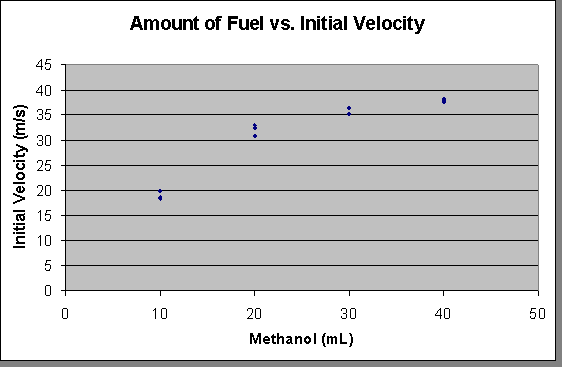
To find the initial velocity of the projectile (as proposed), the range formula was used to determine the velocity based solely from the distance traveled. Since the trajectory angle was known (a constant 45 degrees), the formula used to find the velocity is:
V = v ((range) (g) / Sin (2θ))
Thus the chart, using the initial velocity:
*** Link To Raw Data ***
And the consequent graph:
Conclusion .:. Top
Our findings support our original hypothesis that if the amount of methanol gas used to fuel the projection of a ball through a cannon is increased, then the initial velocity will gradually increase in a logarithmic pattern. The shape of a trend line that could be drawn through the averages of the initial velocities supports our interpretation of a logarithmic relationship:
While a mathematical limit does not exist, the restrictions upon the physical materials used to build our cannon significantly curb the relationship. This “virtual” limit (near 45 m/s) would most likely be a function of the volume of gas within the fuel chamber that is being ignited. This tapering-off effect most likely exists because, as the energy of the explosion increases, more energy is waited in sound energy, heat energy, etc. Also since there is no infinitely hard material to built the cannon out of, some energy may have escaped actually expanding/bending the firing chamber. Since the volume the methanol has to combust in is constant, the more methanol used, the more energy was wasted in slowly expanding the firing chamber. It is for these reasons we feel the relationship between fuel and velocity was curbed.
The possible errors that existed within the experiment play a key role in determining the relationship between the two variables. The error of greatest concern would be the saturation of the atmosphere within the fuel chamber. This is to say that it is not necessarily the amount of fuel injected within the fuel chamber that is essential. Rather, it is how saturated the atmosphere inside of the chamber is with methanol. The clear difference between the amount of liquid fuel and gaseous fuel in the chamber must be noted, due to their respective chemical volatility when ignited. The variations in saturation levels can depend upon several human errors. These factors include: the pressure at which the spray bottle was squeezed, the angle at which it was squeezed, the location from which the alcohol was injected, the volume of the chamber (depending upon the placement of the projectile within the chamber, due to the fact that it serves as an endcap for the firing chamber), and the intensity of shaking the chamber to cause the alcohol to saturate the atmosphere. Under ideal conditions, these errors could be eliminated or at least improved. Instead of using a mister or spray bottle to inject the fuel, the fuel could be directly poured into the fuel chamber, and either allowed to sit until it naturally evaporates (saturating the atmosphere), or aided through the use of a direct heat application to the exteriors of the fuel chamber. Though either of these method would be more effective, much more time would be consumed in conducting each test. Furthermore, the saturation level of the fuel chamber would have to be assumed, seeing that no direct inspection would reveal the exact saturation level.
Other errors, some not human-controlled in comparison to the errors outlined above, include the air resistance, the aiding or hindering effects of the wind (whether holding the projectile back, or causing a greater initial velocity), and whether or not the ground we fired off of was perfectly level. However, despite the various factors which affect the outcome of the experiment, the data collected was sufficient enough to base a solid conclusion off of, supporting our hypothesis outlined at the beginning of the experiment. What must be considered is that when building a practical cannon, there exists no set of procedures that results in a perfectly efficient cannon. Because of this, we feel that our data accurately represents what would be the results of firing a more meticulously designed, industrial-proof methanol cannon.
Related Sites .:.
Top
Safety (MSDS) data for methyl alcohol.
http://physchem.ox.ac.uk/MSDS/ME/methyl_alcohol.html
Article on Methanol From Wikipedia.
http://en.wikipedia.org/wiki/Methanol
Yet another Wikipedia entry about the history of the cannon.
http://en.wikipedia.org/wiki/Cannon
A website about the history of projectile motion.
http://library.thinkquest.org/2779/
Information on a depressing methanol cannon accident at a school.
https://carnot.physics.buffalo.edu/archives/1998/11_1998/msg00403.html
Bibliography .:. Top
1) Giancoli, Douglas C. Physics: Principles with Applications. Prentice Hall, 1998
2) Lewis Sr., Richard. Hawley's Condensed Chemical Dictionary. John Wiley & Sons, 1993
3) Oxford University Chemistry Lab. "Safety (MSDS) data for ethyl alcohol." 2004
4) Trefil, James. Encyclopedia of Science and Technology. The Reference Works Inc., 2001
5) Smith, M.G., and M. Snyder. "Ethanol-induced virulence of Acinetobacter baumannii". American Society for Microbiology meeting. 2005
6) Unknown, Projectile Motion. 26 Oct. 2005
<http://library.thinkquest.org/2779/History.html>.


