Method
Bibliography
Return to
research
Background information
Of course, the importance of
projectile weapons is to get an object to go a long distance with as little
effort as possible. To get the optimal use out of your weapon, you want to make
sure that you can get the perfect balance of range and power. You can get
something to go a long ways, but it will take a lot of power. If you do not
have enough power, then it will not go anywhere. In addition, if you have too
much power, then you can blow up the weapon you are using to fire the object.
For example, in a model rocket, if the engine you use is too big, then the
rocket can blow up.
Statement of problem
The purpose of this experiment is to
find the optimal amount of methanol to use in the bottle to produce the most
efficient launch distance and power. I will change the amount of ethanol in
order to obtain this effect.
Review of literature
Ballistics in general has to do with the launching
of projectiles from different weapons. A projectile is “any object that is cast,
fired, flung, heaved, hurled, pitched, tossed, or thrown. The path of a
projectile is called its trajectory” (Hart, 1). For a long time, many in war
and science have used ballistics and projectile weaponry. Ballistics, in the
time ofarrows, started when “Niccollo Tartagila described a quadrant, which was
an instrument inserted into the gun to measure the angle of the barrel” (Toma,
1). It has progressed a lot over the years; in fact, it has progressed to the
point at which we can make very sophisticated bullets used for many different
purposes. Including, “soft point for maximum damage, hard point for maximum
penetration, and many in between” (encyclopedia.com). Many people have done
experiments with different vapors, including, “Michael L. Corradini and Riccardo Bonazza who are experimenting with the
effects of cold water and molten metal that combust to form vapor explosions”
(engr.wisc.edu). Lastly, an experiment with liquid ethanol vapor in which, “the
ethanol is ignited to form an explosion inside the bottle and forces the stopper
off” (sprott.physics.wisc.edu).
Hypothesis
I believe that the launch velocity will
increase proportionately with the amount of ethanol in the bottle untill a
point at which it will level off and rise slowly, if at all. The amount of ethanol
is defined as the number of drops put into the bottle. The launch velocity is
defined as {(distance/time)^2+((9.8*time)/2)^2}^.5 TOP
In my experiment, I tried to keep my
method as precise as possible, and I also tried to keep it consistent
throughout to limit the amount of variation between launches. I would start by
dropping in however many drops were required for that experiment, and then I
would put the cork on the top. I tried using a rubber stopper, but there was
too much friction between it and the bottle, so it did not launch at all. After
I put the cork on the top, I would shake the bottle for about 15 to 20 seconds
to vaporize as much of the methane as possible. Then I would strap it into the
launching device with a small bungee  chord as shown in the picture below. After I have
placed the bottle securely on the launch device; which my dad and I built with
our bare hands (and a few power tools), I would use the high frequency generator (zap
thing) to ignite the methane and launch the cork. As soon as it launched, I
would start my watch and then stop it when the cork hit the ground. This is
where I would encounter my worst error to be discussed about later. I would
watch the cork very carefully in order to see where it landed. I would the
measure from that point until directly under the launching device. I would then
dry out the inside of the bottle with silica gel packets so it would launch
again. After a little bit, my lovely assistant (my mom) came out and helped me
by drying out the bottle while I used another bottle exactly like it. We figure
this saved me about two and a half hours of experimentation.
chord as shown in the picture below. After I have
placed the bottle securely on the launch device; which my dad and I built with
our bare hands (and a few power tools), I would use the high frequency generator (zap
thing) to ignite the methane and launch the cork. As soon as it launched, I
would start my watch and then stop it when the cork hit the ground. This is
where I would encounter my worst error to be discussed about later. I would
watch the cork very carefully in order to see where it landed. I would the
measure from that point until directly under the launching device. I would then
dry out the inside of the bottle with silica gel packets so it would launch
again. After a little bit, my lovely assistant (my mom) came out and helped me
by drying out the bottle while I used another bottle exactly like it. We figure
this saved me about two and a half hours of experimentation.
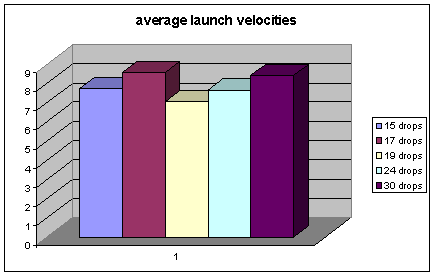
In the end, my results came back inconclusive,
because I had trouble being perfectly accurate in some of the steps in my
experiment. What did occur was in some ways concurrent with my hypothesis, yet
some aspects were not. As you can see, 17 drops in the bottle experienced the
highest velocity, yet all the others were relatively in order. In each of the
different variables, the launch velocity varied greatly, as shown below. Some
of them varied by as much as 3.5 m/s.
|
# of
drops |
diff. in
launch velocities (m/s) |
|
15 |
2.433095366 |
|
17 |
3.322939716 |
|
19 |
1.751300273 |
|
24 |
3.073475141 |
|
30 |
3.514964532 |
As I stated before, my results were inconclusive,
yet I know what I could do next time, especially if I had a bigger grant. My
biggest error that I encountered was the inconsistencies in my timing. Part of
it was my slow reactions, which I could fix by rigging up a device to start the
time when the cork leaves the bottle then stop it when the cork hits the
ground. Of course, this is not feasible to most people, so I recommend having
two or more people timing, then average it out. It would also help if the
experiment was performed inside a building so you would not have to worry about
wind. As well, there has to be a way to regulate how much ethanol vapor is
inside the bottle, because there is no way to regulate that in the way I was
performing the experiment. One thing I noticed from just messing around was
when I used a bigger bottle it shot much higher than when I put similar amounts
of ethanol into them. I think that this is because there is more area for the
ethanol to vaporize into, so there are more reactants to propel the cork. I also did not notice much variation between
the numbers of drops, so that is why my last two were at larger variables,
because I was hoping for some better change. I think that if I had done that
through the whole experiment, I would have noticed my distinct variations. It
also took a while for me to find an amount at which it would launch, and I finally
found that 15 drops did it. In the end, I am pleased with my experiment as a
whole, and I had fun doing it.
Bibliography (in
order of use) TOP
http://www.hypertextbook.com/ by Glen Hart, 5:15 10/25/04
knowledge on the mechanics of bullets.
http://www.tomacorp.com/ by Toma, 4:53 10/25/04
http://www.encyclopedia.thefreedictionary.com
by unnamed, 5:27 10/25/04
www.engr.wisc.edu/
by unnamed, 5:36 10/25/04
http://www.sprott.physics.wisc.edu/
by Mr. Sprott, 5:39 10/25/04
