The Effect of Temperature on the Viscosity of Corn Syrup
Manasi Sridhar
January 25, 2019
IB Physics Internal Assessment
Viscosity is defined as the property of resistance to flow in any material with fluid properties.[1] Thinner, free flowing fluids like water and milk are relatively less viscous. Thicker, more sluggish fluids like corn syrup and glue are more viscous. They have more resistance to flow. Viscosity can be calculated with Stokes’ Formula:
F = 6pi*n*r*v
where F is force in Newtons, n is the viscosity of the fluid, r is the radius of the sphere dropped in the fluid in meters, and v is the velocity of the sphere in the fluid.
Statement of Question: The purpose of this investigation is to find the relationship, if any exists, between the temperature and viscosity of corn syrup.
Hypothesis: I believe that as temperature increases, the viscosity of the corn syrup will decrease. This will happen because as the temperature of the fluid rises, the kinetic energy of its molecules also will increase. The fluid will become less ‘sluggish’ and ‘flow better’.
Method: To set up this experiment, I acquired a graduated cylinder, a glass thin cylindrical tube, a permanent marker, corn syrup, a cooking thermometer, a timer, two corks, and a tiny steel ball. I filled the graduated cylinder with 150 mL of water at different temperatures. I marked 9 cm on the thin cylindrical tube, and filled it completely with corn syrup. I used the two corks to keep the corn syrup inside the tube. Then, I placed the corn syrup filled tube in the water and let it sit until the corn syrup was the desired temperature. I dropped the steel ball into the tube and started my timer when the ball passed the first marking. I stopped the timer when the ball passed the second marking. I repeated this procedure for 5 trials for each temperature.
Here are two photos of my setup. I filled the graduated cylinder to 150 mL for each trial for the 9 temperatures. The tube filled with corn syrup was closed off with two corks.


Here is a close up of the tube filled with corn syrup; the metal ball is circled. I used the same metal ball and corn syrup for every trial and every temperature.

Reasoning behind experimental design:
To ensure the success of my experiment, I had to regulate all the variables, so that the only aspect that was changing was the temperature. I held the volume of the water to 150 mL, used the same thermometer, corn syrup, glass tube, timer, distance for the ball to travel, and the same ball itself. By controlling these variables, I was able to eliminate any other outside effects on the experiment. For the actual experiment itself, I used 9 different temperatures varying in intervals of 10 degrees Celsius, in order to see the gradual change in viscosity as temperature increases. I did 5 trials and 9 temperature variants to find many data points. This was necessary to make a valid correlation.
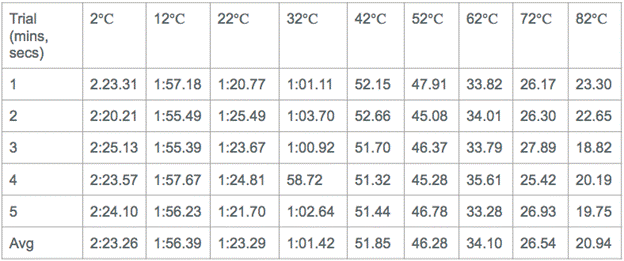
Calculated Data:
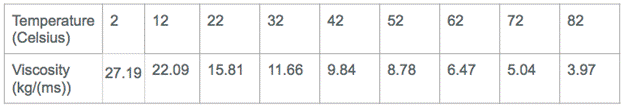
Explanation of Calculations: I used Stokes’ Formula to calculate the viscosity of the corn syrup at each temperature. The way I did this was by calculating the buoyant force of the metal ball I dropped into the corn syrup and setting that equal to (6pi*n*r*v). I measured the radius of the ball. I found the velocity by dividing the .09 m that the ball traveled by the average times that corresponded to each of the 9 temperatures. I did this for each temperature.
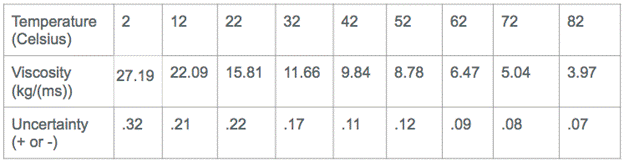
Discussion of uncertainty:
The human component of this experiment makes it prone to error. The biggest uncertainty arises with the timing of the ball as it traveled the 9 cm through the corn syrup. It is not logical to believe that I was 100% accurate when starting and stopping the timer as the ball passed the markings on the glass tube. The uncertainty accounts for the slight discrepancies here.
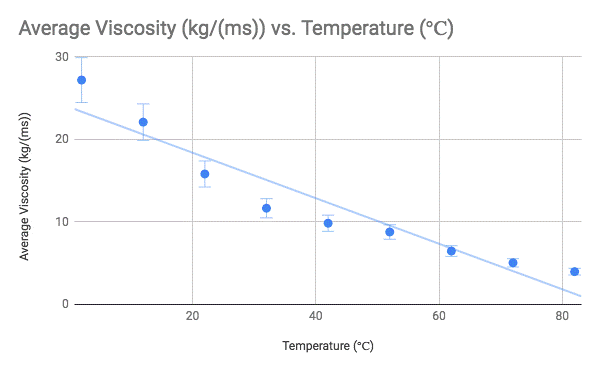
Conclusion:
After analyzing my data, I have concluded that there is a pattern in the relationship between temperature and corn syrup viscosity. In the experiment, as the temperature increased, the viscosity of the corn syrup decreased. In other words, there is an inverse relationship between temperature and viscosity. Although my hypothesis was correct, and there was a strong negative correlation between temperature and viscosity, I was not expecting the drop in viscosity to be so drastic as the cold temperatures became warmer, and lesser as the hot temperatures got warmer. Though I was able to draw a line of best fit, it could be argued that the relationship between temperature and viscosity is actually an example of exponential decay.
One flaw in my experimental design, was the reliance on my own eyesight and perception. The timing of the metal ball as it descended the 9 cm in the corn syrup very likely was not completely accurate each time, due to simple human error. Also, there could have been some misreading of the thermometer, as the lines are tiny and very close together. Both of these errors in the experiment do not have simple fixes, as they were committed by me, the experiment designer and conductor. A way to offset the error is to just do more trials (10 trials per temperature). Another way to offset the error is to do more variations in temperature. Though this is true, I feel that 9 temperatures was sufficient to show a correlation between temperature and corn syrup viscosity.
If I were to redo this experiment, I would try more variations and trials, but I would also try different fluids. I think an interesting take on the experiment I did would be to see which fluids lose their viscosity the fastest.
https://physics.info/viscosity/
- This source is good because it explains viscosity in depth, and the site explains how to measure viscosity.
https://resources.saylor.org/wwwresources/archived/site/wp-content/uploads/2011/04/Viscosity.pdf
- This source gives graphs and explains the relationship between viscosity and temperature on a molecular level.
http://www.columbia.edu/itc/ldeo/lackner/E4900/Themelis3.pdf
- This source is a textbook on physics from Columbia University that describes viscosity and its varying factors in detail.
- This source first introduces the reader to viscosity, then it gives an exercise that measures viscosity, and there is a informative video on viscosity, too.
https://www.britannica.com/science/viscosity
- This is a good source because it gives a simple encyclopedia definition of viscosity while also providing enough detail to be useful.
Elert, Glenn. “Viscosity – The Physics Hypertextbook.” Free Fall – The Physics Hypertextbook, physics.info/viscosity/.
“Viscosity and Stokes Equation.” Ucsc Physics Demo, 7 June 2017, ucscphysicsdemo.wordpress.com/physics-5b6b-demos/viscosity-and-stokes-equation/.