The Effect of Water
concentration in Isopropyl Alcohol on Heat Energy Production by Combustion
Candidate number: gsk094
Table of Contents
The Effect of Water concentration in Isopropyl Alcohol on Heat Energy Production by Combustion
Introduction:
Fire has long fascinated mankind, from the Greek myth of
Prometheus bringing fire to mankind from Mount Olympus to the common
representation of fire as synonymous with life, and has long been the focus of
many scientific studies; parallel to this is the study of flammable materials
and their properties. Keeping in line with mankind's age-old pyromania I
investigated the effect of adding different amounts of water to isopropyl
alcohol and set it ablaze and measured the change in temperature, in order to
determine the amount of heat energy produced. My experiment uses
thermodynamics, specifically the formula for the production of heat energy ![]() . This formula finds the
heat energy (Q), by multiplying the mass of the substance (m), by the specific
heat of the substance (c), and by the change in temperature (
. This formula finds the
heat energy (Q), by multiplying the mass of the substance (m), by the specific
heat of the substance (c), and by the change in temperature ( ![]() ). The source that I used for my experiment was Recycle
USA inc, for the weight of the aluminum in a soda can.
). The source that I used for my experiment was Recycle
USA inc, for the weight of the aluminum in a soda can.
Experiment
Outline:
Research
Question:
What effects do adding different amounts of water to isopropyl alcohol have on the energy production through combustion?
Hypothesis:
I hypothesize that as the concentration of water increases, then the heat energy production will decrease.
Variables:
In my experiment, the independent variable was the amount of water added to the isopropymal alcohol, with the isopropyl alcohol being a constant 2 ml. The dependent variable was the temperature of the water (40 ml) in the can suspended over the burning isopropyl alcohol-water mixture.
Materials:
- One aluminum can
- One ring stand, ring, and mesh
- One pewter bowl
- One container of Isopropyl Alcohol (99% concentration)
- Two 40 ml beakers
- One glass pipet (6ml)
- Matches
- A timer
- A pair of tongs
- A bucket of water
- A fire extinguisher (safety first)
- Google sheets
- T-84 calculator
Set
up:

Procedure:
In order to conduct my experiment I first set up my ring
stand, complete with ring and mesh to hold my can of water (40 ml) that
was to be heated, placing a thermometer into the mouth of the can in order to
measure the temperature of the water. I then filled the 40 ml beakers full of
water, as well as the bucket and prepared the pewter bowl under the can. I then
used the pipet to measure and place 2 ml of isopropyl
alcohol into the pewter bowl, followed by the amount of water, starting at 0 ml
and increasing in intervals of .2 ml. After mixing the solution as evenly as
possible, I measured the initial temperature of the water in the can, I then readied my timer and used a match to light the
solution and started the timer, and then put the spent match in the second
beaker of water. I watched the solution burn and stopped the timer when it put
itself out and recorded the final temperature of the water in the can. I then
used the tongs to move and cool the pewter bowl, and the can (with the water
inside) with the bucket of water (being careful not to spill any water from the
can, or add any from the bucket to the can0. I repeated this process until I
reached a mixture of 3 ml of water and 2 ml of isopropyl alcohol (1 trial per
solution), and then entered them into google sheets.
I then recorded all of my raw data and organized it, then proceeded to use google sheets to calculate the change in temperature, used
my calculator to calculate the percentage of water in the solution
![]() . I then used google sheets to calculate the heat energy produced by each
solution, using the formula
. I then used google sheets to calculate the heat energy produced by each
solution, using the formula ![]() ; with m1 is the
mass of the 40 ml of water in the can, c1 is the specific heat of water (4.1855
J/g oC),
; with m1 is the
mass of the 40 ml of water in the can, c1 is the specific heat of water (4.1855
J/g oC), ![]() being
the change in temperature for each solution (in both parts of the formula), m2
is the mass of the aluminum in the can (14.9 g), c2 is the specific heat of
aluminum (0.902 J/g oC), and Q is
the heat energy produced.
being
the change in temperature for each solution (in both parts of the formula), m2
is the mass of the aluminum in the can (14.9 g), c2 is the specific heat of
aluminum (0.902 J/g oC), and Q is
the heat energy produced.
Raw
Data:
|
Amount of Water (ml) |
Initial temperature |
Final Temperature |
Change in Temperature |
|
0 |
23 |
86 |
63 |
|
0.2 |
58 |
103 |
45 |
|
0.4 |
43 |
104 |
61 |
|
0.6 |
21 |
81 |
60 |
|
0.8 |
45 |
94 |
49 |
|
1 |
55 |
100 |
45 |
|
1.2 |
45 |
82 |
37 |
|
1.4 |
54 |
94 |
40 |
|
1.6 |
42 |
82 |
40 |
|
1.8 |
46 |
90 |
44 |
|
2 |
42 |
86 |
44 |
|
2.2 |
38 |
77 |
39 |
|
2.4 |
39 |
84 |
45 |
|
2.6 |
40 |
80 |
40 |
|
2.8 |
36 |
81 |
45 |
|
3 |
37 |
80 |
43 |
Processed
Data:
In order to convert my raw data into usable data, for my temperature I subtracted my initial temperature from my final temperature, in order to calculate the change in temperature. Ex. 86-23=63. Additionally in order to calculate the percentage of water in each solution I took the amount of the total solution and divided it by the amount of water added. Ex. 2.2/.2=9.09%.
Finally to calculate the heat energy produce, I utilized the
formula ![]() ; with m1
is the mass of the 40 ml of water in the can, c1 is the specific heat of water
(4.1855 J/g oC),
; with m1
is the mass of the 40 ml of water in the can, c1 is the specific heat of water
(4.1855 J/g oC), ![]() being the change in temperature for each solution (in
both parts of the formula), m2 is the mass of the aluminum in the can (14.9 g),
c2 is the specific heat of aluminum (0.902 J/g oC),
and Q is the heat energy produced. Ex. Q=40*4.1855*63+14.9*0.902*63, Q=11394.1674.
being the change in temperature for each solution (in
both parts of the formula), m2 is the mass of the aluminum in the can (14.9 g),
c2 is the specific heat of aluminum (0.902 J/g oC),
and Q is the heat energy produced. Ex. Q=40*4.1855*63+14.9*0.902*63, Q=11394.1674.
Graph
1:
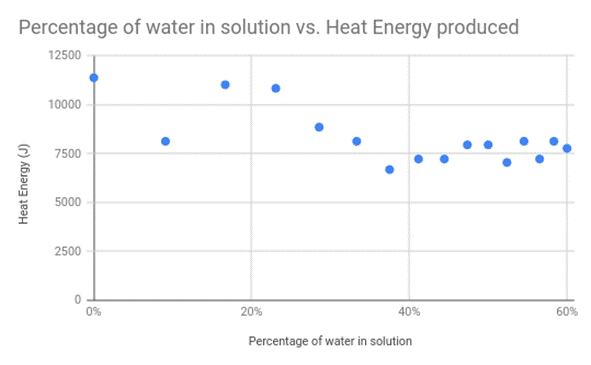
Graph 1 represents the amount of heat energy produced (J), over the percentage of water in the solution. Graph 1 shows that as the percentage of water increases the heat energy produced decreases to a point, then begins to level out.
Table
1:
|
% of water in solution |
Q/ Heat energy (J) |
|
0% |
11394.1674 |
|
9.09% |
8138.691 |
|
16.67% |
11032.4478 |
|
23.07% |
10851.588 |
|
28.57% |
8862.1302 |
|
33.33% |
8138.691 |
|
37.50% |
6691.8126 |
|
41.17% |
7234.392 |
|
44.44% |
7234.392 |
|
47.36% |
7957.8312 |
|
50% |
7957.8312 |
|
52.38% |
7053.5322 |
|
54.54% |
8138.691 |
|
56.52% |
7234.392 |
|
58.33% |
8138.691 |
|
60% |
7776.9714 |
Table 1 represents the information given in graph 1 in a tabular form, showing that as the percentage of water increases the heat energy produced decreases to a point then begins to level off.
Table
2:
|
% of water in solution |
Change in T |
|
0% |
63 |
|
9.09% |
45 |
|
16.67% |
61 |
|
23.07% |
60 |
|
28.57% |
49 |
|
33.33% |
45 |
|
37.50% |
37 |
|
41.17% |
40 |
|
44.44% |
40 |
|
47.36% |
44 |
|
50% |
44 |
|
52.38% |
39 |
|
54.54% |
45 |
|
56.52% |
40 |
|
58.33% |
45 |
|
60% |
43 |
Table 2 represents the relationship between the percentage of water in the solution and the change in temperature, which is proportional to the relationship between the percentage and the heat energy produced.
Conclusions
and Evaluation:
Conclusion:
The heat energy produced, after a certain percentage of water in the solution, begins to lower and eventually reaches a consistent amount.This conclusion partially supports my hypothesis, as it initially decreases, however my hypothesis did not predict the leveling off of the decrease as the percentage increased. This conclusion is supported by the findings of graph 1, which displays the trend depicted in the conclusion, as well as the data found in table 1.
Evaluation:
One of the weaknesses of this study were the low number of trials, with multiple trials for each concentration serving to strengthen the data set. Another weakness of the experiment is the non-digital thermometer, which could have been affected by the speed at which I read the temperature. This lack of precision meant that I could only measure in whole degrees, without decimal places, meaning that my calculations could be slightly off. If I were to conduct this experiment again I would conduct more trials for each concentration (2-3) and I would employ a digital thermometer connected to a computer, in order to collect more accurate and precise temperatures.
Related
Websites:
https://recycleusainc.com/how-many-aluminum-cans-equal-1-pound/
Summary: provides the mass for the aluminum can used in the experiment.
https://en.wikipedia.org/wiki/Combustion
Summary: explains the processes and types of
combustion in a fairly easily digestible fashion.
https://webhome.phy.duke.edu/~rgb/Class/phy51/phy51/node59.html
Summary: Explains the first law of
Thermodynamics and the formulas used within the experiment.
https://www.grc.nasa.gov/www/k-12/airplane/combst1.html
Summary: Provides an explanation for combustion
generally and as it pertains to rockets.
https://en.wikipedia.org/wiki/Heat
Summary: provides general information on heat
and gives a basic understanding of the methods of heat transfer.