PreQuiz 15.1 - PV Diagrams .:. Go Up
The Formulas:
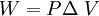
W = work done changing the volume in J
P = Pressure in Pascals (1 Pa = 1 N/m2) Gauge pressure (PG) is the amount of pressure more than one atmosphere. P = PG + 1 ATM
DV = change in volume in m3.
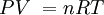
P = the absolute pressure in Pa. Don't use gauge pressure. If you get pressure in psi, or any other unit, and or gauge, convert it to Pa absolute:
4.0 psi gauge = 4.0 psi + 14.7 psi = 18.7 psi absolute (because 1 atm in psi is 14.7) and finally, 18.7/14.7 = P/1.013x105, so P = 126,797.2789 Pa absolute.
Remember, 1 atm = 1.013x105 Pa = 101.3 kPa = 14.7 psi = 760 Torr.
V = Volume in cubic meters. (m3) There are 1000 liters in 1 m3.
n = number of mols. n = mass/molar mass.
R = 8.31JK-1mol-1
T = Absolute temperature in KELVINS. T = oC + 273.15
The Prequiz:
This is a Pressure v Volume graph for 0.28 mols of an ideal gas.
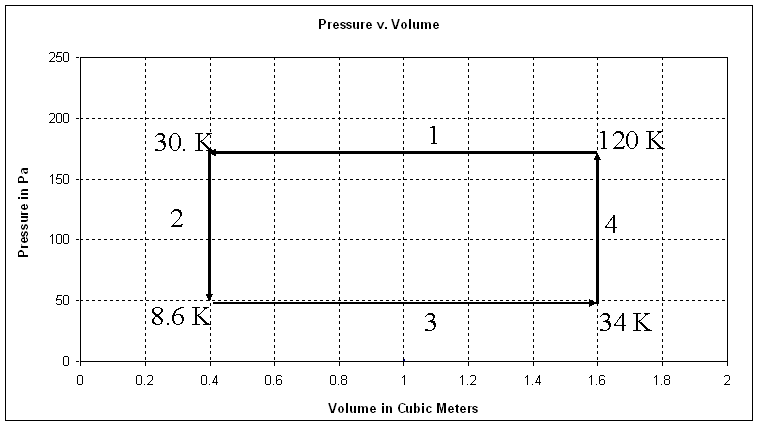
The system starts at a pressure of 175. Pa and a volume of 1.60 m3 and goes through these four processes:
- Isobaric (constant pressure) compression to 0.40 m3
- Isochoric (constant volume) cooling to 50. Pa
- Isobaric expansion to 1.60 m3
- Isochoric heating to 175 Pa
Draw all four processes. Use arrows for the processes, and label each process 1, 2, 3 or 4
1. Calculate the temperature (in K) at the end of each process. Label these temperatures at the corners on the graph above.
Starting at the beginning of cycle 1:
P = 175 Pa, V = 1.60 m3:
PV = nRT, (175 Pa)(1.60 m3) = (0.28 mols)(8.31 J/mol K)(T) - the mols are given above the graph
P = 175 Pa, V = 0.40 m3:
PV = nRT, (175 Pa)(0.40 m3) = (0.28 mols)(8.31 J/mol K)(T)
P = 50.0 Pa, V = 0.40 m3:
PV = nRT, (50.0 Pa)(0.40 m3) = (0.28 mols)(8.31 J/mol K)(T)
P = 50.0 Pa, V = 1.60 m3:
PV = nRT, (50.0 Pa)(1.60 m3) = (0.28 mols)(8.31 J/mol K)(T)
2. Which processes have Zero net work done? Why is no work done? (Answer in words)
2 and 4 – the volume does not change
3. Which processes have non-zero work? What is the work done by each process that has non-zero work?
Processes 1 and 3 have a change in volume, so we can use
to calculate the work:
Process 1: W = (175 Pa)(0.40 m3 - 1.60 m3) = -210. J (Negative because the change in volume was negative)
Process 3: W = (50.0 Pa)(1.60 m3 - 0.40 m3) = + 60.0 J
4. What is the net overall work done by this cycle?
Um -210 J + 60.0 J = -150. J