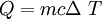
Prequiz 14.1 - Heat and Calorimetry .:. Go Up
The Formulas:
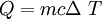
Q = heat added in Joules
m = mass in kg
c = specific heat in J/kgoC. THis is the heat needed to raise one kg one oC
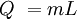
Q = heat added in Joules
m = mass in kg
L = Latent heat of fusion (melting) or vaporization (boiling) in J per kg
The Prequiz:
Show your work, and circle your answers and use sig figs to receive full credit.
This is a graph of temperature v. heat added for a .218 kg sample of unknown. It starts as a solid, and ends as a gas.
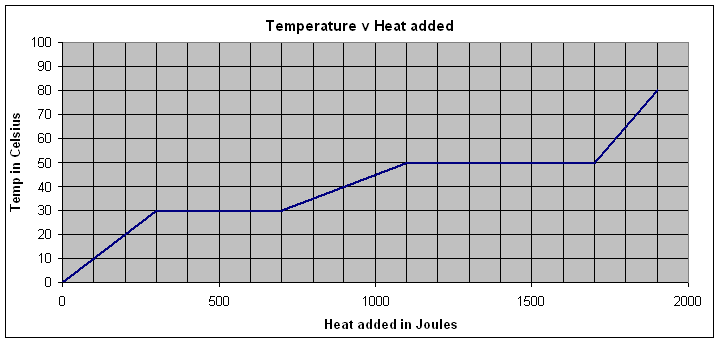
1. What is the melting temperature and the boiling temperature of this substance? Label the solid, liquid and gaseous phases.
(Melt = 30 oC, Boil = 50 oC, solid 0-300 J added, Liquid 700-1100 J added, Gas 1700-2000 J added.)
Where the temperature is rising, it is all one phase (i.e. solid, liquid, gas). Where the temperature plateaus, the substance is undergoing a phase change - either melting, or boiling.
2. What is the latent heat of fusion? (Melting)
(1830 J/kg)
Use
Q = 400 J (starts at 300 J, ends at 700 J)
m = 0.218 kg (see above the graph)
L = 1834.86 = 1830 J/kg
3. What is the specific heat of the liquid phase?
(91.7 J/kgoC)
Use
Q = 400 J (starts at 700 J, ends at 1100 J - Read the graph)
m = 0.218 kg (see above the graph)
DT = 20 oC (starts at 30oC ends at 50 oC - Just read the graph)
C = 91.743 = 91.7 J/kgoC
4. What heat do you need to heat 3.29 Kg of water at 21.0 oC to steam at 175 oC? (For H2O: Cice = 2100 J/kgoC, lf = 3.33 x 105 J/Kg, Cwater = 4186 J/KgoC, lv = 22.6 x 105 J/Kg, Csteam = 2010 J/kgoC)
(9,019,350.76 J – 9.02 x 106 J)
This will need to heat to boiling from 21.0 oC to 100.0 oC (The boiling point of water), then turn to steam, then heat from 100.0 oC to 175 oC. Add all the heats up.
First - use
m = 3.29 kg
DT = 79 oC (starts at 21.0oC ends at 100 oC )
C = 4186 J/kgoC (for the liquid)
Q = 1,087,983.26 J
Next use
m = 3.29 kg
L = 22.6 x 105 J/Kg (vaporization - it's boiling)
Q = 7,435,400 J
Next - use
m = 3.29 kg
DT = 75 oC (starts at 100oC ends at 175 oC )
C = 2010 J/kgoC (steam)
Q = 495,967.5 J
Finally - add them all up:
1,087,983.26 J + 7,435,400 J + 495,967.5 J = 9,019,350.76 J = 9.02 x 106 J
5. 500. grams of a mystery liquid at 45 oC is mixed with 300. grams of water (C = 4186 J/KgoC) initially at 22 oC. The final temperature of the mixture is 33 oC. What is the specific heat of the mystery liquid? (Assuming no heat was lost to the surroundings)
(2,302.3 – 2.30 x 103 J/kgoC)
Calorimetry problems are
m1c1DT1 = m2c2DT2
Sometimes there is more than one term on a side, but the left side is for the hot things that lose heat, and the right side is the things that gain the heat:m1 = .500 kg
c1 = ??
DT1 = 45 - 33 = 12 oC
m2 = 0.300 kg
c2 = 4186 J/kgoC
DT2 = 33 - 22 = 11 oC