A Study of “Bouncy Balls:”
By Matt Sorlien
Background | Hypothesis | Procedure | Data Analysis | Conclusion | Works Cited | Links | Author
Background | Table of Contents
In 1924, Herman Staudinger discovered the basic “code” for polymers, after which it became increasingly easy for chemists to create different types (Osswald). Basic polymers are materials made of molecules of very high molecular weight; known as macromolecules. Some polymers can be extremely hard, others soft and flexible,
while still others are elastic and “gummy.” Many polymers can be formed into a certain shape, then melted and reformed. One special division of polymers is elastomers (Osswald).
Elastomers are elastic polymers that exhibit a “snap” or “rebound” when stress is applied and released (Billmeyer). The molecules in elastomers are cross-linked with each other, forming an intricate grid off bonds. This permits “full extension” of the material (Osswald). These links give the elastomers the ability to deform up to 400%, yet the cross-linking prohibits the individual molecules from sliding past each other (Osswald), thus making them resistant to breakage (NSTA). The two most common artificial elastomers (rubber being a natural one) are Isobutylen – isoprene – and Chloroprene – neoprene (Lynch). They can undergo immense strain, even under low and high temperatures (Osswald). As a result of these properties, they are used for many different commercial and industrial applications. These and other elastomers like them are also used in the production of a common toy referred to as a “bouncy ball.”
Hypothesis | Table of Contents
With the evidence presented and the fact that many polymers become more flexible when heated, I hypothesize that the elastomer will bounce higher when in a higher than room temperature condition.
Procedure | Table of Contents
First, I secured a meter stick to the counter next to the oven to mark the drop point for the ball. Then I dropped the ball from the top of the meter stick and estimated the height of the bounce (this is done on a smooth, hard tile floor). Next to the meter stick, I stacked books on a chair to the approximate height of the initial bounce (73cm), then placed a small board on top which extended into the area where the ball would fall through. This board acted as an aide the process of estimating the height of each bounce. After this first test bounce, the trials commenced.
First, the ball was placed inside the oven, which was set at a designated temperature, for about 45 minutes prior to the trial.Once the ball was at the correct temperature, it was removed from the oven with metal tongs (to reduce heart transfer from my hands to the ball). Then I would slide the board out of the way, and drop the ball from the top of the meter stick. After the ball had passed the position of the board, but before it had bounced back up from the floor, I would slide the board back into the path of the ball (this took coordination). This made it easier to see what height the ball had actually bounced to. After the first drop, I would reposition the height of the board so it was closer to the actual bounce height, then drop again. I would reposition the board again (if needed) then return the ball to the oven for about 5 minutes to ensure that it was at the correct temperature. Then it was removed and three recorded drops were
conducted. Then the ball was placed back into the oven to heat to a different temperature.
Data points
were taken for every 2 degrees from 0C
to 60C and 100C.
Due to the lack of appropriate equipment, I was unable to take data for
62C to 98C.
Data Analysis | Table of Contents
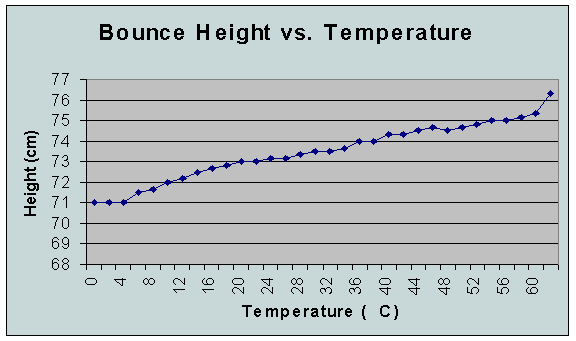 This
increase in bounce height could be from the slight loss of rigidity in
the cross-linking between the molecules of the polymer. This more flexible
state would add to the ability of the substance to deform and snap back
into its original shape, thus
This
increase in bounce height could be from the slight loss of rigidity in
the cross-linking between the molecules of the polymer. This more flexible
state would add to the ability of the substance to deform and snap back
into its original shape, thus
leading to a higher bounce.
The data point for 100C is placed directly after 60C to represent the amount of change for the extrapolated data (62C to 98C).
This big of a change in the performance of the polymer may be important. If the amount of “rebound” is decreased/increased as much as it is, then the polymer may be unstable and prone to breakage at more extreme temperatures. This means it would
probably not be the best polymer to use in industrial processes (maybe that’s why they used it to make a bouncy ball!). It may also be important that the data from 0C to 6C came out the same, which could be a clue that temperatures colder may not effect the polymer as much (more extensive testing is needed to answer that).
Raw Data File (.txt format) | Data
Conclusion | Table of Contents
In addition, a better measuring system would improve the accuracy of the data. Although I used the board method to improve my chances of seeing the actual bounce height, it is truly impossible for the human eye to be able to see where it really was. Some kind of laser measurement device would perform better than my contact-aided eye.
Also, the
ball itself is inconsistent. It was manufactured in two pieces and then
put together. During the “put it together” stage, the machines slid the
slides a little off, so a ridge runs along the ball. This could lead to
slightly stray bounces that make the bounce height lower than its potential.
A more uniform ball would help to reduce stray bounce. Lastly, the type
of polymer used in the bouncy ball is obviously made for low stress uses.
The type of polymer used greatly effects the results, and I was not able
to find out what they used (and I tried), then these results only represent
that unknown type
of polymer.
Due to these facts, the is a notable uncertainty in the experiment. I estimate the measurement uncertainty at as much as .5cm, taking into account all the factors. Even through these faults and irregularities in the experiment, the fact remains, the ball did bounce higher when under increased temperature, just as I had predicted. Further experimentation with better equipment and more types of bouncy balls could further establish this point.
Works Cited
| Table of Contents
Lynch, Charles T. Practical Handbook of Material Science. © 1989. CRC Press, Inc. Boca Raton, Florida.
Mark, James (ed). Science and Technology of Rubber. © 1994. Academic Press, Inc. San Diego, California.
National Science Teachers Association. Polymer Chemistry. © 1995. NSTA, Alington, Virginia.
Osswald, Tim. Materials Science of Polymers for Engineers. © 1996. Hanser/Gardner Publications, Inc. Cincinnati, Ohio.
Links | Table of Contents
American Chemical Society: Division of Polymer Chemistry Great source for information about Polymer Chemistry
Society of Chemical Industry Another great source for information on Polymer Chemistry
The Polymer Technology Group, Inc. See polymers at work! This site gives you a look into the world of polymers as useful materials in industry
American Institute of Chemical Engineers A general site about chemical engineering, of which Polymer Chemistry is a part
Chemical Resources brought to you by UC Berkeley. All the links you could possibly want, and more!!
Matt Sorlien was born in 1982 in Berkeley, California. His family moved to Tualatin, Oregon seven years later. There he attended Tualatin High School and took two years of physics under the ever-popular, master of teaching, Mr. Murray. Matt loved physics class, and even got the opportunity to create a web page about his Physics II research paper.
Matt also enjoys reading, computers, watching sports (it really doesn’t matter what), tennis and swimming. He started swimming competitively swimming when he was 8. Eventually, he became very dedicated to the sport and started to go to as much as 11 practices per week. He has, as of 5/26/00, attended three national championships. His highest placing was 14th in the 200m backstroke (twice). He also recently qualified to swim for the National Junior Team and will be competing in England in 2001.
In September 2000 he will continue his education at Stanford University in Palo Alto, California. He is considering studying Chemical Engineering. He will also be swimming on the team.
Here are more links: