What is temperature’s effect on surface tension?
Table of Contents
Personal Engagement .:. Top
The decision to do a presentation on surface tension was a bit of a simple one. I knew that it was something unique to my class’ experiments and I knew that I could implement it in a physical experiment rather than a simulation, which to me seemed favorable. I found a picture online of what I wanted my experiment to look like and was determined to make it a reality. I was planning on making a scale to test my experiment and, with the help of my grandfather; we were able to make the actual scale that I used in my experiment. Overall, I enjoyed adding my own personal touches to this surface tension experiment.
Exploration .:. Top
Within this IA, I hope to answer the question: What is temperature’s effect on surface tension?
Background Information
Surface tension is essentially the tension on the surface of a liquid which is caused by the attraction of the bonds on the surface. So, as some may know, when a liquid is heated or cooled then the molecular bonds change. When it is hot they move faster and farther apart which causes weak bonds, while when it is cold they slow down, move closer together, and create strong bonds. Looking at this information I thought that this must have some sort of effect on surface tension. To see this effect in action I did research to determine what I thought was the best method of collection. After some time I decided on the scale, or balance option. Below is a picture of my final scale.

Procedure
Essentially, the way that is scale measures surface tension is simple. First things first, since the needle and the counterweight are very different weights, I needed to add a piece of red clay to the top of the scale to ensure that it was initially balanced. From there, I take the needle, which is hanging from the right hand side by a thin thread, and gentle place it into a bowl of water. This bowl of water was changed out quite frequently for different trials to either change the temperature of the water or to replenish the water so that I could maintain a semi-consistent temperature. To measure the temperature I used a laser heat reading gun. For my counterweight I had a foil box on the other side and I would use it to contain the staples that I would drop into it. Basically, I am trying to add just enough weight to the other side of the scale so that the needle will be ‘ripped’ off the surface of the water. I chose to use staples because they were small enough that I could add multiple before the scale began to tip. This way, my measurements could be a bit more precise. At first, I began with room temperature water, which was the easiest temperature to maintain and came out around 19° C. I dropped the needle in, slowly added staples, and recorded how many it took to remove the needle from the water. After I repeated that 10 times I moved on to the next temperature of water which was what I considered cold (2-5° C). I used water that I had kept in my freezer since the beginning of the experiment which had begun to form a bit of ice around the edges. Needless to say, this water was cold. Again, after the ten trials I moved on the hot water (66-68° C). This was the most difficult type of water because it was the hardest temperature to maintain. I used an insulated thermos to try to keep it warm, which helped, but I still had to keep a pot of water boiling on the stove so that I could replenish the water whenever it got too cool. Again, I repeated the ten trials and recorded the data. By calculating the amount of staples that it took to remove the needle from the water, I could find surface tension by using the equation S=F/2d. S being surface tension, F being force, and d being the length of the needle (.037 m). To find the force I used F=mg, with the mass of the staples being .0666 g for one staple multiplied by the average amount of staples. So you can see how I was able to find the surface tension of a liquid through this process.
Safety Concerns
The only major safety concern of this lab is that boiling water was used. I was very conscious of this and made sure not to burn myself around it. It was especially important to be aware of this because this was the water temperature that I had to replenish most often so there was always a pot of boiling water on the stove.
Analysis .:. Top
Firstly, it is important to look at the data of how many staples it took to remove the needle from the water. Below is my data table showing this for the differing water temperatures along with the average, the force that I calculated, and the accompanying graph.
|
Number of Trials |
19° C (room temp) |
2-5° C (cold) |
66-69° C (hot) |
|
1 |
11 |
14 |
11 |
|
2 |
13 |
14 |
11 |
|
3 |
12 |
13 |
11 |
|
4 |
13 |
13 |
10 |
|
5 |
12 |
12 |
11 |
|
6 |
12 |
13 |
10 |
|
7 |
13 |
12 |
11 |
|
8 |
13 |
13 |
11 |
|
9 |
12 |
13 |
10 |
|
10 |
12 |
12 |
11 |
|
Average |
12.3 |
12.9 |
10.7 |
|
Force (mg) |
.0080441992 N |
.0084365992 N |
0.00699773 N |
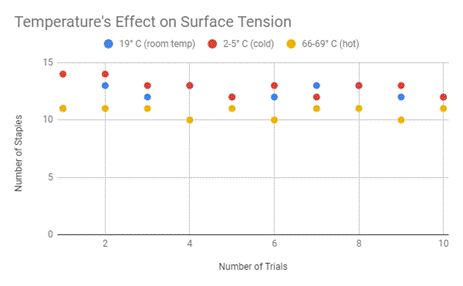
Then from there and with the data that was collected, I was able to calculate the surface tension using the equation, S=F/2d, which was described in the above section.
|
Temperature of Water |
Surface Tension |
|
19° C (room temp) |
0.1087053946 N/m |
|
2-5° C (cold) |
0.114008097 N/m |
|
66-69° C (hot) |
0.0945639189 N/m |
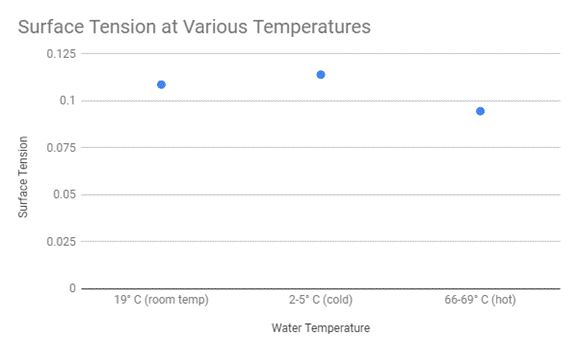
Evaluation .:. Top
Conclusion
As seen by the data above, we can come to the conclusion that temperature does affect surface tension. While the room temperature and cold water had similar outcomes, when taking into account the scale of the experiment, the hot water had a noticeable difference in surface tension. This can be justified fairly simply. As mentioned beforehand in my background information, when a liquid heats up the bonds move faster and farther apart which weakens the bonds within the liquid. This is what happened here. When the water was heated to almost boiling temperatures, this weakened the bonds and made it harder for the needle to latch on like it did in the other two cases. This is very similar to what the scientific community also concluded. According to the University of Florida, “In general, surface tension decreases when temperature increases because cohesive forces decrease with an increase of molecular thermal activity” (fsz.ifas.ufl.edu). They then modeled this conclusion with this graph.
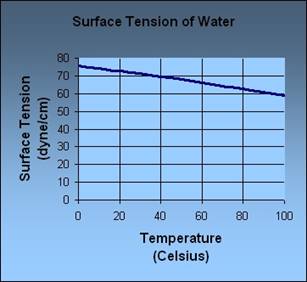
So it can be concluded that while this experiment did have its errors, I was able to come up with a conclusion that was very similar to that of the scientific community.
Strengths and Weaknesses
While I did end up getting the results that I hoped for, there were still many weaknesses and errors within this experiment. We’ll start with the biggest one: There was a considerable difference in the surface tension of the hot water, but I was unable to create the adverse effect with the cold water. I think that this may have been because my scale was not 100% accurate. It is a homemade scale so this is to be seen. I think that the major pitfall of the scale’s accuracy was at its pivot point. There was too much resistance between the wooden crossbeam and the screw that it rested on for it to move entirely freely. There was also the issue of trying to keep a consistent temperature. Not only would I have to change out water quite frequently, but it also caused me to move quite quickly through the process perhaps missing important details or not letting the scale fully balance out between staples. All things considered, I still think that this was a fairly accurate experiment and one that was not hard to recreate at home.
Suggested Improvements
If I were to do this again I would set out to improve in two areas: the scale and the temperature maintenance. In regards to the scale, I would want to put some sort of metal coating in the drill hole, maybe some sort of lubricant, really anything that might have reduced the friction at the pivot point. My grandfather and I initially intended to do this but we could never find the right material. I would also try to contain the temperature better. I don’t know how easily this would be to accomplish at home however. I think that the best way to contain the temperature may be to put foil on top of the clear cup and make a slit small enough for the needle to enter the cup. This way, the top of the cup, where the majority of the heat was lost, would be more enclosed and the heat would have a harder time escaping.
Related Websites
· http://fsz.ifas.ufl.edu/surfacetensionandcapillarity/html/en_tension.htm - This website was great because it had graphs that specifically showed the effects of temperature on surface tension.
· https://www.wikihow.com/Measure-Surface-Tension - This website was helpful in giving me different ideas in how I can conduct my experiment.
· https://www.khanacademy.org/science/biology/water-acids-and-bases/cohesion-and-adhesion/v/surface-tension - This gives a great general overview of surface tension as a concept.
· https://www.youtube.com/watch?v=v7TYZ4CcO2E – This video briefly discussed the theory behind why temperature affects surface tension.
· https://www.education.com/science-fair/article/viscosity-surface-tension-temperature/ - This website gives another great example of how to complete the same experiment but in a different way.
Work Cited .:. Top
- "Tension Superficial." Surface Tension. Accessed January 25, 2019. http://fsz.ifas.ufl.edu/surfacetensionandcapillarity/html/en_tension.htm.