Research
Question: What is the effect of the specific heat of various substances on the
temperature of hot coffee?
Table of
Contents:
Introduction | Hypothesis | Variables | Equipment | Methodology | Trials | Conclusion | Evaluation | Related Websites | Go to Top
In this investigation, I plan to
determine and record how the specific heat of various substances affects the
temperature of hot coffee. I thought of this due to a common problem I run into
on a daily basis; since I have to go to school almost every day, I rarely have
time to wait for my coffee to cool down. Due to this, I was struck with a
question when my class began studying thermodynamics; how can I use specific
heat to solve my daily issue? When two fluids of different temperatures are
mixed, the resulting fluid must eventually balance out. This is why, for
example, pouring cold coffee into a hot cup of coffee will slightly cool the
coffee; the mixture is reaching an equilibrium between the two temperatures.
The resulting temperature of the coffee/milk mixture is lower than that of the
coffee alone, but higher than that of the milk alone. There are many variables
that go into what exactly this final temperature will be, such as the
temperatures of the respective substances, the mass of the substances, and the
specific heat of the substances. Since different fluids have different specific
heat, this has led me to the final question I aim to answer: what is the
relationship between the time it takes to reach a temperature equilibrium and
the specific heat of fluids?
I plan to use ten different fluids, those being milk,
apple juice, pulpless orange juice, water, soda, lemonade, cranberry juice, coconut
water, fruit punch, energy drink. My independent variables will be the time it
takes for the mixture to cool, while my dependent variable will be the specific
heat of the substances. My control variables will be the starting temperatures
of everything and the mass. I believe that, because of how easy it is to heat
and cool water, this will be the one that cools the coffee the fastest.
Hypothesis: The higher the specific heat of the substance, the cooler it
will make my coffee when they are mixed.
Scientific Reasoning: The specific heat is the
amount of heat required to raise the temperature by one degree Celsius.
Therefore, a lower specific heat would heat faster. Inversely, something with a
higher specific heat would heat up faster. By finding the specific heat of
things usually added to coffee, I can determine which is more effective by
comparing their specific heats and finding the one with the highest specific
heat.
Independent Variable:
●
Substance being used
Dependent Variable:
●
Specific heat (found using the formula mcΔt=mcΔt)
Controlled Variables:
●
Amount of boiling water (250 mL)
○
Reason for controlling variable: consistency, an attempt to
ensure there aren’t too many errors in the data
○
How it will be controlled: the boiling water will be measured
before being mixed.
●
Temperature of water (83 degrees Celsius)
○
Reason for controlling variable: consistency
○
How it will be controlled: I will attempt to pour the water
directly into the measuring cup after being boiled so little heat is lost.
●
Amount of substance being tested (50 mL)
○
Reason for controlling variable: consistency
○
How it will be controlled: the substance will be measured out
to 50 mL before being mixed.
●
A calorimeter built from the following materials:
○
One paper cup
○
One thermos with a lid and a small hole in that lid
○
A thermometer
●
6750 mL total of boiling water
●
150 mL of coffee
●
150 mL of milk
●
150 mL of sugar
●
150 mL of agave blue syrup
●
150 mL of coconut oil
●
150 mL of beer
●
150 mL of wine
●
150 mL of Pepsi
●
150 mL of A1 steak sauce
●
Electric kettle
●
Measuring cups
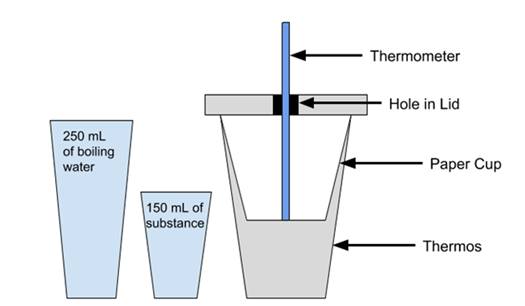
Fig
1. The set up (not pictured: the electric kettle that the water is boiled in,
timer)
- Set water to boil (this will
take a few minutes)
- Measure out 150 mL of substance
being tested
- Measure temperature of substance
- Write down temperature
- Pour out water once it has
boiled into 250 mL measuring cup
- Pour water into thermos and
screw on lid (so less heat escapes)
- Measure temperature of water
- Write down temperature
- Pour in substance
- Measure temperature immediately
after mixing
- Write down temperature
- Repeat above process three times
per substance
- Calculate the specific heat for
each trial using mcΔt=mcΔt with the first mcΔt being water
(250*4.186*(Initial temp - final temp)) and the latter mcΔt being the
substance (150*c*(Final temp - Initial temp)) and solving for c
○
Example: (250*4.186*(80-60))=(150*c*(60-40))
■
c=6.97666667 KJ/kg
- Find the average specific heat
for each substance
- Compare the average specific
heats
- Conclude and evaluate
Trials for Milk
|
|
Initial
Temperature of Milk |
Initial
Temperature of water |
Final
Temperature |
Specific
Heat |
|
Trial
1 |
9 |
77 |
69 |
2.790666667 |
|
Trial
2 |
10 |
83 |
70 |
4.050967742 |
|
Trial
3 |
10 |
84 |
69 |
5.321186441 |
Average Specific Heat: 4.054273617
KJ/kg
Trials for Coffee
|
|
Initial
temperature of coffee |
Initial
temperature of water |
Final
temperature |
Specific
heat |
|
Trial
1 |
46 |
83 |
77 |
4.186 |
|
Trial
2 |
48 |
83 |
78 |
3.805454545 |
|
Trial
3 |
49 |
85 |
79 |
4.330344828 |
Average Specific Heat: 4.107266458
KJ/kg
Trials for Sugar
|
|
Initial
temperature of sugar |
Initial
temperature of water |
Final
temperature |
Specific
heat |
|
Trial
1 |
23 |
85 |
75 |
3.949056604 |
|
Trial
2 |
22 |
86 |
75 |
4.263518519 |
|
Trial
3 |
23 |
85 |
76 |
3.6225 |
Average Specific Heat: 3.945025041
KJ/kg
Trials for Coconut Oil
|
|
Initial
temperature of coconut oil |
Initial
temperature of water |
Final
temperature |
Specific
heat |
|
Trial
1 |
22 |
85 |
76 |
3.488333333 |
|
Trial
2 |
22 |
87 |
77 |
3.805454545 |
|
Trial
3 |
22 |
86 |
77 |
3.424909091 |
Average Specific Heat: 3.57289899
KJ/kg
Trials for Agave Blue Syrup
|
|
Initial
temperature of agave |
Initial
temperature of water |
Final
temperature |
Specific
heat of agave blue syrup |
|
Trial
1 |
21 |
86 |
74 |
4.651111111 |
|
Trial
2 |
21 |
87 |
75 |
4.566545455 |
|
Trial
3 |
21 |
87 |
75 |
4.566545455 |
Average: 4.594734007 KJ/kg
Trials for Beer
|
|
Initial
temperature of beer |
Initial
temperature of water |
Final
temperature |
Specific
heat of beer |
|
Trial
1 |
22 |
81 |
75 |
2.462352941 |
|
Trial
2 |
22 |
85 |
76 |
3.6225 |
|
Trial
3 |
22 |
82 |
75 |
2.872745098 |
Average Specific Heat: 2.985866013
KJ/kg
Trials for Wine
|
|
Initial
temperature of wine |
Initial
temperature of water |
Final
temperature of mixture |
Specific
heat of wine |
|
Trial
1 |
19.5 |
85 |
73 |
4.784 |
|
Trial
2 |
20 |
86 |
76 |
3.805454545 |
|
Trial
3 |
19.5 |
86 |
76 |
3.704424779 |
Average Specific Heat: 4.097959775
KJ/kg
Trials for Sunflower Oil
|
|
Initial
temperature of sunflower oil |
Initial
temperature of water |
Final
temperature of mixture |
Specific
heat of sunflower oil |
|
Trial
1 |
20 |
83 |
78 |
1.835964912 |
|
Trial
2 |
18 |
85 |
82 |
1.00464 |
|
Trial
3 |
20 |
85 |
82 |
1.0465 |
Average Specific Heat: 1.295701637
KJ/kg
Trials for A1 Steak Sauce
|
|
Initial
temperature of A1 steak sauce |
Initial
temperature of water |
Final
temperature of mixture |
Specific
heat of A1 steak sauce |
|
Trial
1 |
10 |
85 |
71 |
4.764552846 |
|
Trial
2 |
10 |
86 |
72 |
4.651111111 |
|
Trial
3 |
10 |
89 |
75 |
4.5784375 |
Average Specific Heat: 4.664700486
KJ/kg
Trials for Pepsi
|
|
Initial
temperature of Pepsi |
Initial
temperature of Pepsi |
Final
temperature of mixture |
Specific
heat of Pepsi |
|
Trial
1 |
22.5 |
87 |
76 |
4.470485437 |
|
Trial
2 |
22.5 |
88.5 |
77 |
4.498971963 |
|
Trial
3 |
22.5 |
89 |
78 |
4.22440367 |
Average Specific Heat: 4.397831367
KJ/kg
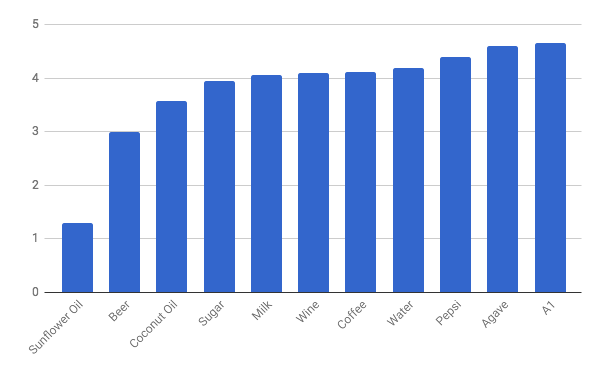
fig
2. Chart comparing the specific heats of the substances tested
As evidenced by the chart above, it
seems as though A1 steak sauce would be the most effective at cooling anything
with a lower specific heat than it, which coffee has. However, due to the fact
that this is testing cooling coffee to drink, it would be the most advantageous
and make the most sense to use the agave blue syrup in order to cool coffee.
The least effective thing to use to cool my coffee would be the sunflower oil.
My hypothesis was proven correct the higher the specific heat, the cooler the
mixture would be, as evidenced by the final temperatures I calculated.
Therefore, the higher the specific heat of the substance, the more effective it
will be as quickly cooling coffee.
Although I did do my best to even everything out,
there are many inconsistencies that may have skewed the results. These errors
are primarily human error, the boiling water being varying temperatures, not
all substances being the same temperature, and the slowness of the temperature
to drop when the ingredients were mixed. If I were to repeat this experiment,
I’d try to get all the ingredients to the same temperature first prior to
mixing them so there would be a consistent temperature. I’d also get a better
thermos, since I suspect heat may have escaped through the hole in the top of
the thermos.
This is a link to a simple calorimetry problem
that is about coffee, like the problem I was solving for my experiment.
http://chemcollective.org/activities/autograded/115
Both of the above sites are about the specific heat of coffee. The one
directly above is another problem about the specific heat of coffee, which is
again what I myself was solving.
https://www.engineeringtoolbox.com/specific-heat-capacity-d_391.html
The above is a list of the specific heats of many different materials,
which is something I made myself by finding the specific heats of many
different materials.
http://theengineeringmindset.com/specific-heat-capacity-of-materials/
The above is another list of the specific heats of many different
materials, which again is what I myself was making when finding and writing
down the specific heats of the materials I was using.
http://www2.ucdsb.on.ca/tiss/stretton/database/specific_heat_capacity_table.html
The above site is yet another list of specific heats, which is something
I had to create myself while trying to find the specific heats of my materials.