Anneka Owens
1/21/16
IB Physics IA
Discharge Rates and Their Effect on Battery Efficiency
Table of Contents
Since the batteries creation in 1799 it has gone through many transformations to get to where it is today. At this day and age we use batteries in most of our devices to give us the convenience of non-tethered power. The modern day batteries made up of typically a metal or plastic tube or a rectangle with two terminals, one positive one negative, on the ends. Inside, a cathode connects to the positive end and the anode connects to the negative, these electrodes are where the chemical reactions that produce the electricity occur (Battery Information). In between them there lies a separator which allows electrical charge to pass freely while keeping the cathode and anodes electrons separate. When a circuit within the battery is complete, the electricity is produced by a series of electromagnetic reactions. The anode experiences an oxidation reaction which creates electrons, while the cathode experiences a reduction reaction which absorbs them (Krieger). When this process is completed electricity is produced and the collector conducts the charge outside of the battery through the load (Brain).
Different batteries and their voltages can produce different amounts of power and batteries can operate at a variety of discharge rates (Brain). The discharge rate of a battery can be controlled by a resistor - a device having a designed resistance to the passage of an electric current. However, the resistance of a resistor is not constant, it steadily increases as the temperature increases. This is because the hotter a material is, the faster the atoms or ions move and that makes it more difficult for electrons to pass through, which translates to a higher electrical resistance (Woodford). Similarly, the rate at which you discharge a battery has great effects on the battery itself, and will alter the charge released from a battery. This is because batteries produce power through the use of chemical reactions, and the rate at which you discharge a battery will affect itís temperature, which in turn will affect the chemical reactions occurring within the battery. The hotter a battery or itís surrounding temperature is, the faster the reactions will occur, and the battery will produce electricity more quickly; however, it will decrease the battery life. And the colder it is, the slower the battery will produce electricity, but the longer it will last (BU-501: Basics About Discharging). The independent variable will be the different resistors while the dependant variable will be the battery life. I believe that the slower the battery is discharged due to its resistor, the less efficient the battery will be, because as the chromium wire resistor is working hard to slow the current the resistor and the battery will lose a portion of charge through the inefficient chemical reactions occurring within the battery (Battery Performance Characteristics).
Design
How does the discharge rate of a battery affect the total energy yielded from that battery?
In this experiment 5 batteries were discharged at varying rates by using chromium wire as a resistor until they were dead. The circuit was made up of alligator clips, the battery holder, an ammeter and chromium wire, and monitored via LoggerPro which took data every 10 seconds until completion.
Set-Up:

Below is a list of the variables involved:
|
Variable |
Type |
How measured/controlled |
|
Charge |
Dependant |
Measured through LoggerPro |
|
Total Energy |
Dependant |
Measured through LoggerPro |
|
Discharge Rate |
Independant |
Controlled through resistor |
|
Current |
Dependant |
Controlled through a resistor and measured by an ammeter |
|
Resistance |
Independant |
Measured by using chromium wire |
|
Time |
Dependant |
Measured through LoggerPro |
Process Steps:
I will measure the length of Chromium wire needed for the desired resistance and attach alligator clips set distance apart. I well then have LoggerPro monitor the battery and take data every 10 seconds as it discharges until the battery is dead. I will then examine the area underneath the curve to find the total energy released and repeat with the remaining 4 trials.
Materials:
● 5 AA 1.5V Kirkland Alkaline batteries
● 1 Ammeter
● Alligator clips
● 1 Battery holder
● 1 Spool Chromium wire
● Computer
● LoggerPro application
Trial #1

Current = .1685A
Trial #2
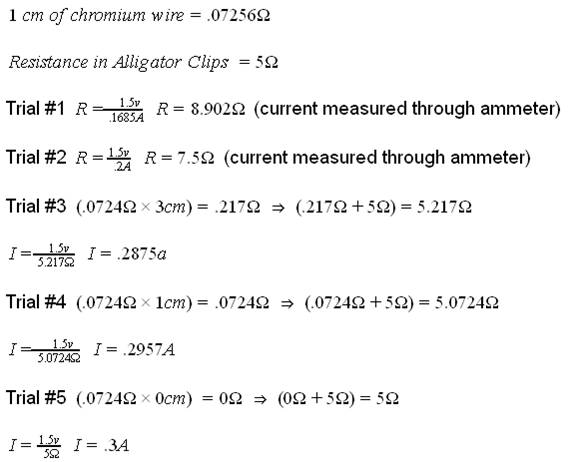
Current = .2A
Trial #3
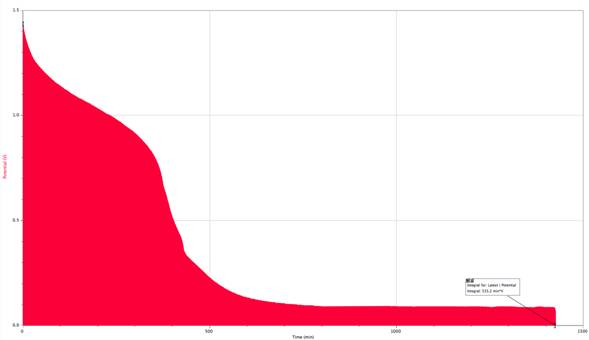
Current = .2875A
Trial #4
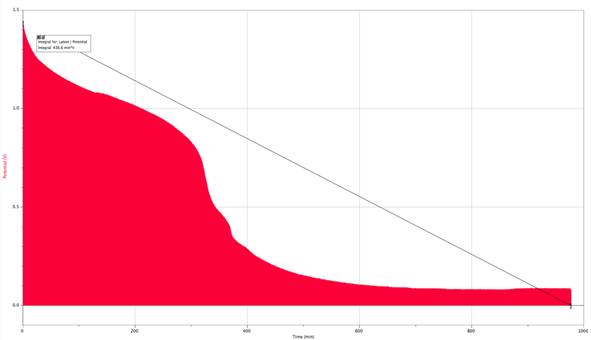
Current = .2957A
Trial #5
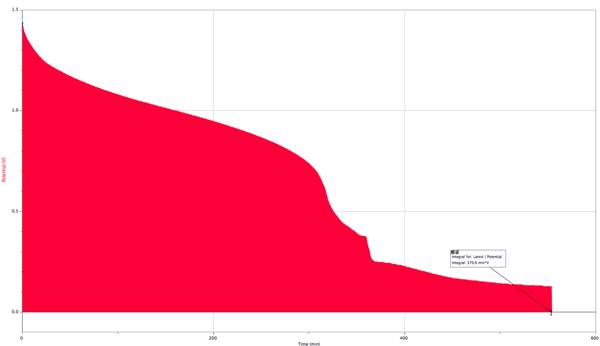
Current = .3A
Raw Data:
|
Battery |
Current (amps) |
Resistance (ohms) |
Time (min) |
Charge Yielded |
|
#1 |
0.1685A |
8.90207Ω |
3010.60 |
201.863 A/s |
|
#2 |
0.2A |
7.5Ω |
1305.08 |
149.733 A/s |
|
#3 |
0.2875A |
5.217Ω |
1425.2 |
102.204 A/s |
|
#4 |
0.2957A |
5.072Ω |
977.0 |
86.08 A/s |
|
#5 |
0.3A |
5.0Ω |
553.5 |
74.12 A/s |
Data file: Excel .:. text: Trial 1 .:. Trial 2 .:. Trial 3 .:. Trial 4 .:. Trial 5 .:.
Calculations:

Error:
There were multiple possible sources of error within this experiment. Firstly, the ammeter only read up to 200mA and because of that I needed to bypass the ammeter and measure the chromium wire for resistance and then use that value to calculate current for trial #3 - #5. The problem with measuring a section of chromium wire for resistance was that the alligator clips were not as precise as other methods. I knew I needed 1cm or 3 cm; however, it was difficult to measure that down to precisely the values needed. So, some of the resistance may have been higher or lower than originally calculated.
In addition, the data was being taken in the basement of my house which has varying temperatures throughout the day. During the day the house is around 68-70 degrees and at night it drops to around 60. I believe that 10 degree variance throughout the trial is responsible for the occasional bumps in the graph. As the house changed from warm to cold the batteries worked better or worse, and that is seen in the sudden drops or rises in the graphs.
My results supported my original hypothesis. The slower I discharged a battery, the more charge was yielded from that battery. The battery with the slowest current of .1685A had 201.863 A/s of charge while the battery with the fastest current of .3A had 74.12 A/s. Nearly halving the current increased the charge yielded by 272.4%. I believe this is due partially to heat loss from the battery and the resistor itself, but majorly to the way heat affected the batteries ability to produce charge. Since the faster current caused the battery to work faster and heat up, the chemical reactions occurring within the battery were affected. The reactions were happening too fast within the battery to be completely as productive as they could be. Further research that could be fun, informative, and have a consumer application is to test this theory among battery brands to see if some brands perform better than others overall, and under certain conditions.
Bibliography
Brain, Marshall. "How Batteries Work." HowStuffWorks. N.p., 31 Mar. 2000. Web. 03 Dec. 2015.
"Battery Performance Characteristics." Battery and Energy Technologies. Electropedia, n.d. Web. 2 Dec. 2015.
"Battery Information." Battery Information. PVEducation.org, n.d. Web. 02 Dec. 2015.
Krieger, Elena M., and Craig B. Arnold. "Effects of Undercharge and Internal Loss on the Rate Dependence of Battery Charge Storage Efficiency." Journal of Power Sources 210 (2012): 286-91. Elsevier, 5 Dec. 2011. Web. 2 Dec. 2015.
Woodford, Chris. "Resistors." How Do Resistors Work? What's inside a Resistor? ExplainThatStuff, 6 Aug. 2015. Web. 23 Dec. 2015.
"BU-501: Basics About Discharging." Battery Discharge Methods Ė Battery University. Battery University, 12 Nov. 2015. Web. 21 Jan. 2016.
http://www.mpoweruk.com/performance.htm
Discusses various battery types and how they operate
https://www.princeton.edu/~spikelab/papers/101.pdf
Interesting data on voltage yielded from batteries
http://data.energizer.com/PDFs/temperat.pdf
Visually displays data regarding temperatures affect on battery life