
Energy Content of Wood
Billy Calder & Ben Love
IB Physics II
Research Project
Table of Contents:
Go Up .:. Introduction .:. Method .:. Results .:. Data Files .:. Conclusion .:. Related Websites .:. Bibliography
Introduction .:. Top
Energy can be measured in different ways, one of which is heat[1]. One property of heat is the law of conservation of energy[2], which states that the total energy of an isolated system cannot change—it is said to be conserved over time. In other words, energy can be neither created nor destroyed, but can change form, for instance chemical energy can be converted to kinetic energy in the explosion of a stick of dynamite. Additionally, The transfer of energy as heat is always from the higher-temperature medium to the lower-temperature one, and heat transfer stops when the two mediums reach the same temperature[3]. This can be seen in air flowing from room to room where there is a draft underneath the door.
Since it is known that heat can neither be created or destroyed, it is then implied to what extent certain reactions are efficient. For instance if a pot of water is being boiled on a stove for sterilization, not 100% of the heat being produced by the stove goes into the water; clearly a noticeable amount of heat gets absorbed into the air and also into the pot itself, therefore making the objective of the heat less than 100% efficient[4]. This goes for every heat transfer event, therefore the goal of a science experiment is to control the environment so the efficiency of the process is as high as possible.
We ultimately investigated the relationship between mass and energy through our experiment of testing the energy content of wood. This was done through burning pieces of wood with the same mass under a set amount of water and consequently measuring the difference in temperature after the wood burned out. We utilized the formula Q=mcΔT to calculate the energy present in the wood; (Q), being energy, is equivalent to the Mass, (M), times the specific heat of the material, (C), times the change in temperature of the substance (ΔT)[5]. It is also important to note how an additional mcΔT was required, because the energy of the water also must have been taken into account in order to determine the difference of heat. The final change in energy (Q) was measured in the temperature of the water (°C). The amount of water and wood were controls, the change in temperature was the dependant variable, and the type of wood was the independent variable.
Our hypothesis predicts that the denser woods will transfer a greater amount of heat than the less dense wood (density measured by the ratio of size to weight).
Method .:. Top
First, we cut down all pieces of the
wood to make sure that each one had the same mass (.7g). Then we filled a can
with 30 mL of water and measured the temperature. Then we lit the wood on
fire, and put the wood under the can for as long as it would burn. We then
measured and recorded the temperature and the final mass of the wood. We
repeated this for three trials of each of the five types of wood that we were
testing.


Other Materials:
● Triple Beam Balance
● Knife
● Clamp Stand
● Clamp
● Graduated Cylinder
Results .:. Top
|
Type of Wood |
Trial 1 |
Trial 2 |
Trial 3 |
||||
|
|
|
Temperature (°C) |
Mass (g) |
Temperature (°C) |
Mass (g) |
Temperature (°C) |
Mass (g) |
|
Pine |
Starting |
22 |
0.7 |
22 |
0.7 |
22 |
0.7 |
|
Final |
38 |
0.2 |
36 |
0.2 |
32 |
0.3 |
|
|
Cedar |
Starting |
22 |
0.7 |
22 |
0.7 |
22 |
0.7 |
|
Final |
42 |
0.3 |
37 |
0.2 |
38 |
0.2 |
|
|
Oak |
Starting |
22 |
0.7 |
23 |
0.7 |
22 |
0.7 |
|
Final |
42 |
0.3 |
39 |
0.2 |
45 |
0.3 |
|
|
Maple |
Starting |
22 |
0.7 |
23 |
0.7 |
22 |
0.7 |
|
Final |
39 |
0.2 |
36 |
0.4 |
36 |
0.4 |
|
|
Hemlock |
Starting |
22 |
0.7 |
22 |
0.7 |
22 |
0.7 |
|
Final |
32 |
0.4 |
30 |
0.4 |
31 |
0.3 |
|
|
Type of Wood |
Average Change |
Average Energy Transfer (J) |
Energy Content (J/g) |
|
|
|
ΔT (°C) |
Δm (g) |
||||
|
Pine |
13.33333333 |
0.4666666667 |
1674.4 |
3588 |
|
|
Cedar |
17 |
0.4666666667 |
2134.86 |
4574.7 |
|
|
Oak |
19.66666667 |
0.4333333333 |
2469.74 |
5699.4 |
|
|
Maple |
14.66666667 |
0.3666666667 |
1841.84 |
5023.2 |
|
|
Hemlock |
9 |
0.3333333333 |
1130.22 |
3390.66 |
|
Q=mCΔT
Energy content=E/m
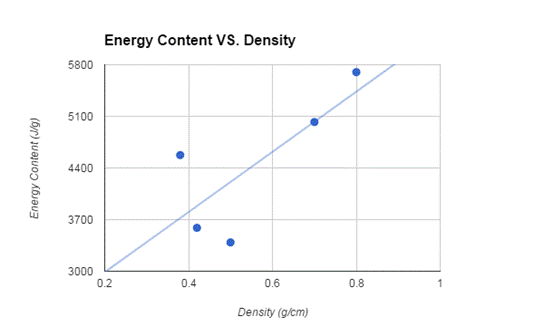
The wood that had the most energy per gram was the Oak wood. The others were as follows: oak, maple, cedar, pine, and hemlock.
Data
Files: Text .:.
Excel .:.
Top
Conclusion .:. Top
The wood in order from most energy per gram to the least was: oak, maple, cedar, pine, and hemlock. The most dense wood to least dense wood was: oak, maple, hemlock, pine, and cedar. The oak and maple followed the order of most dense - most energy, but the hemlock and cedar were switched in the order of the most energy. Overall, though, the two most dense woods did have the most energy.
We hypothesized that the denser woods would transfer a greater amount of heat than the less dense wood, and our findings ultimately supported our hypothesis with the exception of hemlock and cedar. According to research[6], the hardest woods give off the most heat while burning slowly and the softest woods give off the least heat and burned quicker (in terms of density). Our findings put hemlock and cedar out of order according to other research, but proved to match previous data otherwise. This error could have been due to less than optimal samples of available wood, or the age of the wood itself.
One reason why higher density woods produce more heat energy when burned is the greater amount of carbon bonds existing between the atoms of the wood; there is simply more material to burn in a smaller space[7]. But how could the age of the wood have anything to due with the way it burned? Jeff Cordon of the Master Sweep Chimney association explains, “If you have trouble starting your fire, or if you have trouble keeping your fire going, you are probably using this years wood - which means that it's not seasoned. Unseasoned, or green wood, is extremely frustrating and disappointing. If wood is not properly seasoned it will be hard to light. It will keep going out. It will smolder; It won't put out heat. The moisture content in unseasoned wood does not allow the wood to burn well- it just burns poorly and inefficiently. It is also precisely the moisture in wood which causes creosote to build up at an accelerated rate. [...] The moisture content in the wood ultimately determines how much heat the fire puts out”.[8]
The main sources of error would have come from the lack of control of the surrounding environment. There was no way to make all the heat travel directly from the flame to the can, so the heat lost to the air would have been relatively high. The can was made of aluminum which is highly conductive, so it absorbs the heat very quickly and not all of it goes straight to the water. The most likely cause for the cedar and hemlock switching places in the order is that when they were obtained they were very wet, and the cedar was in much smaller pieces so it dried more. This also caused the wood to not stay light for very long which was another cause of heat loss.
To improve this experiment, there should be more control of the environment. A device that insulates the system, and also allows for enough oxygen to fuel the flame would improve the results vastly. The wood would also need to be dried the same amount for each type in order to create an even playing field. It would also help to change the water container and add more water because the temperature was changing by twenty degrees in some cases, and that gives much more reason for errors.
Related Websites .:. Top
http://en.wikipedia.org/
http://www.
http://mb-soft.com/juca/
http://www.
http://www.gfc.state.ga.us/
Bibliography .:. Top
[1] Whyman, Kathryn. Energy and Heat. Mankato, MN: Stargazer, 2005. Print.
[2] Oxtoby & Nachtrieb (1996). Principles of Modern Chemistry, 3rd ed. Saunders College Publishing.
[3] Zhang, Zhuomin M. Nano/microscale Heat Transfer. New York, NY: McGraw-Hill, 2007. Print.
[4] Atesmen, M. Kemal. Everyday Heat Transfer Problems: Sensitivities to Governing Variables. New York: ASME, 2009. Print.
[5] Sustan, C. "How Things Work - Specific Heat." Specific Heat. Physics.csustan.edu, Apr. 2006. Web. 15 Jan. 2015.
[6] Harbison, Michael. Energy Efficiency and Wood-Burning Stoves. EPA. Environmental Protection Agency, Mar. 2011. Web. 15 Jan. 2015.
[7] Harbison, Michael. Energy Efficiency and Wood-Burning Stoves
[8] Cordon, Jeff. Best Wood. Master Sweep Chimney Association, Oct. 2002. Web. 15 Jan. 2015.