Prequiz 13.1 - Ideal Gas Law .:. Go Up
The Formulas:
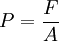
P = Pressure in Pascals (1 Pa = 1 N/m2) Gauge pressure (PG) is the amount of pressure more than one atmosphere. P = PG + 1 ATM
F = Force in N
A = Area in m2. A = LxW for a rectangle, and pr2 for a circle.
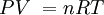
P = the absolute pressure in Pa. Don't use gauge pressure. If you get pressure in psi, or any other unit, and or gauge, convert it to Pa absolute:
4.0 psi gauge = 4.0 psi + 14.7 psi = 18.7 psi absolute (because 1 atm in psi is 14.7) and finally, 18.7/14.7 = P/1.013x105, so P = 126,797.2789 Pa absolute.
Remember, 1 atm = 1.013x105 Pa = 101.3 kPa = 14.7 psi = 760 Torr.
V = Volume in cubic meters. (m3) There are 1000 liters in 1 m3.
n = number of mols. n = mass/molar mass.
R = 8.31JK-1mol-1
T = Absolute temperature in KELVINS. T = oC + 273.15
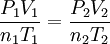
This is not in the data packet - This works if everything is absolute. (i.e. not gauge pressure, not Celcius, not change in mols or change in volume, but absolute mols and volume)
The Prequiz:
(1 atm = 1.013x105 Pa = 101.3 kPa = 14.7 psi = 760 Torr; 1 m3 = 1000 liters; pabsolute = pgauge + 1 atm; )
1. A porthole in an airplane has a diameter of 37.0 cm. If there is a pressure of 412 Torr on the outside of the window, and a pressure of 745 Torr inside, what is the net force pushing out on the porthole? (4770 N)
The area of the porthole is pr2 = p(0.185 m)2 = 0.107521009 m2.
The pressure difference between the inside and outside is 745 T - 412 T = 333 T.
Convert to Pa:
333/760 = P/1.013x105, P = 44,385.39474 PaUse
to find the force:
P = 44,385.39474 Pa, A = 0.107521009 m2, so F = 4772.362408 N = 4770 N(None of the skill set ones is remotely as hard)
2. Fred has 1.65 mols of methane gas at 87.2 oC at 56.3 kPa (1 kPa = 1000 Pa). What is the volume it occupies? (.0878 m3)
P = 56.3 x 103 Pa
V = ?
n = 1.65 mols
R = 8.31JK-1mol-1
T = 87.2 + 273.15
3. Maryland has 217 grams of Neon (molar mass 20.1797 g/mol) gas in 519 liters at gauge pressure of 6.97x104 Pa. What must the temperature be in Celsius? (1000 liters = 1 m3) (720. oC)
P = 6.97x104 Pa + 1.013x105 Pa
V = 519/1000
n = 217/20.1797
R = 8.31JK-1mol-1
T = ? (Subtract 273.15 to get Celcius)
4. An aerosol can is at an absolute pressure of 381 Boogalas when it is at 293 K. If I put it in liquid nitrogen and lower its temperature to 77.0 K, what is the new pressure in Boogalas? (1000 milli Boogalas = 1 Boogala) (Assume it does not leak, and the volume remains constant) (100. Boogalas)
P1 = 381 Boogalas
V1 = Assumed Constant
n1 =Assumed Constant
T1 = 293 KP2 = ??
V2 = Assumed Constant
n2 = Assumed Constant
T2 = 77.0 K
5. A Helium tank contains 3.42 kg of helium and is at a gauge pressure of 145 psi. What will be the gauge pressure when you have released 1.13 kg of helium? (92.2 psi)
P1 = 145 + 14.7 psi
V1 = Assumed Constant
n1 =3.42
T1 = Assumed ConstantP2 = ?? (subtract 14.7 to get gauge)
V2 = Assumed Constant
n2 = 3.42-1.13
T2 = Assumed Constant