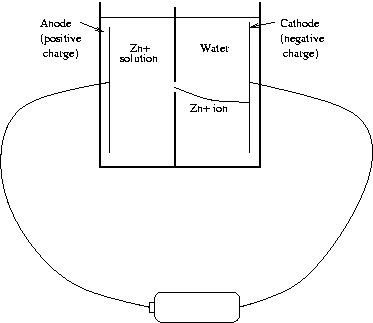
As originally conceived, the experiment was quite simple in principle. We needed a small container with some kind of barrier dividing the inside into two compartments. One compartment would contain the solution with the ions that we wanted to plate, while the other would contain pure water. A small hole would be made in the barrier, allowing the ions to enter the pure water compartment only at a specific location. One electrode would be placed in each compartment, and connected to a battery so that the negative voltage at the cathode would draw the positive ions of the solution through the hole in the barrier and onto itself, producing a small, localized spot. Schematically, the finished product would look something like this.

The experiment, then, would consist of setting up the container with fresh solution and fresh water, connecting the battery, and letting the ions plate for a given amount of time under different g-forces. Then we would determine whether and how far the path of the ions was deflected downward.
In preparation for the actual experiment, we did some cursory research on what materials we should use for the electrodes and for the ion solution. After some initial dead ends, we discovered that two plates of copper with a zinc chloride solution produced a decent effect. We used a 1.5 V battery connected to the two electrodes, and let the zinc ions plate for about 5 minutes. When completed, there was a very visible spot of zinc on the copper cathode, although, unfortunately, it scratched off rather easily. This test run actually consumed quite a bit of time, considering the preparation of chemicals, of our container, and so on. We hoped that with the procedure solidified, the process would go more quickly.