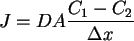
In nature, laws that hold at the macroscopic level are often found to be less and less true on smaller scales. For instance, we intuitively expect water droplets to fall to the earth when thrown in the air, and they usually do. However, the fact that clouds can be formed from tiny evaporated water beads, suspended magically in the air by unseen forces, demonstrates the fact that physics gets complicated on small scales. It is this general statement that we wish to explore.
It's an obvious fact that heavy objects sink. Archimedes' study of buoyancy set forth the physical laws that govern this process. His findings state that an object immersed in a fluid (liquid or gas) will feel an upward buoyant force equal in magnitude to the weight of the displaced fluid. So if the object is denser than the fluid, its weight will be greater than the buoyant force and it will sink [Bartlett].
However, this generalization does not always hold with very small particles. When tiny particles, atoms or molecules, are released in water, they do not follow the laws that hold on macroscopic levels. The particles are susceptible to the random motion of the water molecules, and are bumped and shoved in every direction. The result is the phenomenon called diffusion-the particles spread themselves fairly evenly throughout the container of water, a fact that can be easily demonstrated. Although there are too many particles interacting to formulate absolute laws describing their motion, statistical laws are possible. The rate J of diffusion is given approximately by
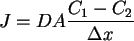
where D is the diffusion constant, A is
the cross-sectional area of the container, and
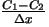 is the concentration gradient across the container [Giancoli].
The D term is affected in a number of ways, and thus affects
the rate of diffusion. For instance, with gases at low pressure, D
increases with temperature and decreases with the mass of the molecule.
However, this is only roughly true in most situations. For liquids,
the only simple statement that can be made is that the rate of diffusion
increases with temperature [Fuller].
is the concentration gradient across the container [Giancoli].
The D term is affected in a number of ways, and thus affects
the rate of diffusion. For instance, with gases at low pressure, D
increases with temperature and decreases with the mass of the molecule.
However, this is only roughly true in most situations. For liquids,
the only simple statement that can be made is that the rate of diffusion
increases with temperature [Fuller].