A STUDY INTO THE EFFECTS OF VELOTIOUS DIHYDROXIDE UPON COOLING
Matt Deckard
Water, the most abundant natural resource on our planet, is a fluid. And as a fluid, it acts in such a way that follows the physics of a fluid. Air, similarly, is considered a fluid by aerospace engineers, though one does not commonly think of it in this fashion. So, theoretically, these two "fluids" should act alike, according to the physics of a fluid. The purpose of this study is to find out what the effect will be in terms of temperature of moving water, at varying speeds, on an object, as opposed to stationary water, with the knowledge that moving air typically cools objects. Air, being comprised of gases, obeys the first law of thermodynamics: energy is conserved. In other words, if a speeding particle collides with another stationary particle which absorbs the impact by exerting an equal outward force, pressure, leaving two stationary particles, then the kinetic energy of the first was either given off as heat into the air, or was absorbed into the internal energy of the second, which should result in an increase in temperature of the second object; since temperature is directly proportional to energy, then as the energy of the second particle increases, so does its temperature. However, when air is moving, like from a fan, it actually cools an object, overall, by "blowing" heat of the surface of an object; in other words, heat was probably released into the air, after expanding towards the objects outer walls, and then carried away from that object, quicker than if it were simply dissipating into the stale air, since, instead, there is a steady flow of cooler air surrounding the object, providing more favorable conditions for entropy, allowing for it to cool via entropy, though faster due to this "blowing"action. Since both water and air are considered to act as "liquids," they should act similarly. Heat should also transfer similarly, according to the laws of thermodynamic--most importanly, that of entropy. Therefore, I hypothesize that the object will become cooler, due to the moving water, as opposed to nothing acting upon the object at all; this response should be further exaggerated with an increase in the speed of the water.
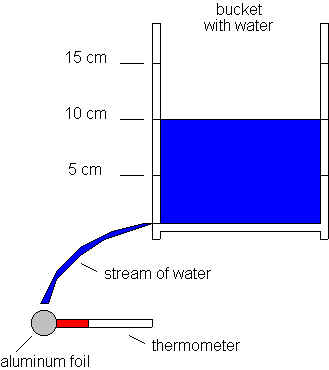
My setup involved a bucket, a thermometer, a watch, aluminum foil, and water. First, I drilled three pairs of holes at heights 5 cm, 10 cm, and 15 cm into the side of the bucket; in addition, I drilled a hole near the bottom corner of the bucket from which I would be measuring the velocity from and using for the bulk of my experimental data. Next, I filled the bucket with water up to 5 cm, plugging the hole at the bottom, and measuring the temperature of the water to be 10 degrees Celsius. Then, I wrapped the head of the thermometer with aluminum foil, of which I would be measuring temperature change, and heated it to close to 40 degrees Celsius (exact measurements are listed). Finally, I held the aluminum foil wad in the path of the stream of water, having removed the plug and having begun constantly pouring water back into the bucket, as to maintain a constant height, and thus, a constant velocity; I proceed to time how long it took for its temperature to decrease to approximately that of the water. I completed these steps five times for each of the 3 heights, taking note of both the horizontal and vertical distances of the stream of water to the aluminum foil. After all this, I concluded the data gathering part of my procedure by emersing the approximately 40 degrees Celsius aluminum foil wad in the water, measuring the amount of time which transpired in order to decrease its temperature to approximately that of the water, as to juxtapose this with my prior results involving the effects of velotious water upon cooling, as opposed to stale water--solely entropy.

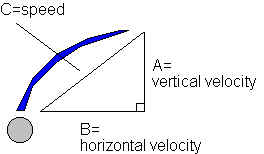
Using the formula v^2=u^2+2*a*s, where the initial velocity u=0 m/s, the acceleration due to gravity a=9.8 m/s^2, and s is the measured vertical distance, I calculated the vertical velocity of the stream, which does not vary among heights. With this velocity, I calculated time, being universally equal among both horizontal and vertical results, with t=s/v; using this time, I calculated the horizontal velocity for each set of results with v=s/t, s being the previously measured horizontal distance. I then found the approximate speed of the hypotenuse of the stream, which is the speed at which the aluminum foil is being struck at, with the geometric formula A^2+B^2=C^2:
Continuing, I found the change in temperature per second for each of the velocities, including the stale water. I then calculated uncertainty by taking the difference between the high and low figures for change in temperature per a second of each of the four groups of results, dividing by two, and using the largest of these numbers as my uncertainty for change in temperature per a second. I also found the average change in temperature per a second for each of the four groups by adding each group of five results together and dividing that number by five, applying my calculated uncertainty to each of these numbers and comparing them, according to velocity, which I was sure of to the nearest centimeter in my measurements, so I disregarded any uncertainty in this; thus, I concluded this experiment and examined the results. (see results)
![]()
temperature of water = 10
vertical distance = 20 cm
horizontal distance = 11 cm

average = -(1.55+1.53+1.88+1.77+1.88)/5 = 1.72
minimum = -1.72+.20 = -1.52
maximum = -1.72-.20 = -1.92
vertical velocity
v^2 = u^2 + 2*a*s
v^2= (0)^2 + 2(-9.80)(.20)
v^2= 3.92
v = -1.98
time
t = s/v
t = (.20)/(1.98)
t = .10 s
horizontal velocity
v = s/t
v = (.11)/(.10)
v = 1.10 m/s
speed
A^2 + B^2 = C^2
(-1.98)^2 + (1.10)^2 = C^2
5.13 = C^2
C = 2.27 m/s
temperature of water = 10 Celsius
vertical distance = 20 cm
horizontal distance = 16 cm

average = -(1.78+1.63+1.94+1.94+1.77)/5 = -1.81 C/s
minimum = -1.81+.20 = -1.61 C/s
maximum = -1.81-.20 = -2.01 C/s
vertical velocity
v^2 = u^2 + 2*a*s
v^2 = (0)^2 + 2(-9.80)(.20)
v^2 = 3.92
v = -1.98 m/s
time
t = s/v
t = (.20)/(1.98)
t = .10 s
horizontal velocity
v = s/t
v = (.16)/(.10)
v = 1.60 m/s
speedA^2 + B^2 = C^2
(-1.98)^2 + (1.60)^2 = C^2
6.48 = C^2
C = 2.55 m/s
temperature of water = 10 Celsius
vertical distance = 20 cm
horizontal distance = 18 cm
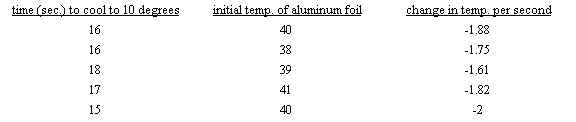
average = -(1.88+1.75+1.61+1.82+2.00)/5 = -1.81 C/s
minimum = -1.81+.20 = -1.61 C/s
maximum = -1.81-.20 = -2.01 C/s
vertical velocity
v^2 = u^2 + 2*a*s
v^2= (0)^2 + 2(-9.80)(.20)
v^2 = 3.92
v = -1.98 m/s
time
t = s/v
t = (.20)/(1.98)
t = .10 s
horizontal velocity
v = s/t
v = (.18)/(.10)
v = 1.80 m/s
speed
A^2 + B^2 = C^2
(-1.98)^2 + (1.80)^2 = C^2
7.16 = C^2
C = 2.68 m/s
temperature of water = 10 Celsius
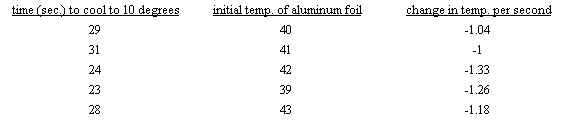
average = -(1.04+1.00+1.33+1.26+1.18)/5 = -1.16 C/s
minimum = (-1.16+.20) = -.96 C/s
maximum = (-1.16-.20) = -1.36 C/s
To find uncertainty, I took the two most extreme figures, high and low, from each group of results, added them together, and divided that number by two. Of the four uncertainties, I used the largest one, as it is the most varying possible. I applied this uncertanty to the average change in temperature per second of each group, finding both the minimum and maximum values at each separate velocity. (see results)
uncertainty of h = 5 cm
(1.88-1.53)/2 = +/- .18
uncertainty of h = 10 cm
(1.94-1.63)/2 = +/- .16
uncertainty of h = 15 cm
(2.00-1.61)/2 = +/- .20
uncertainty of v = 0 m/s
(1.33-1.00)/2 = +/- .17
Change in Temperature per a Second According to Velocity
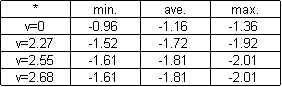
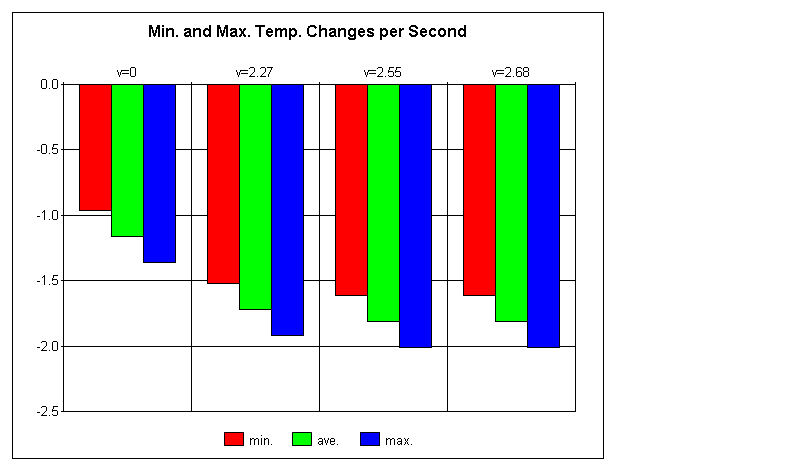
Change in Temperature per a Second According to Velocity
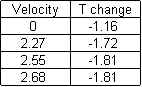
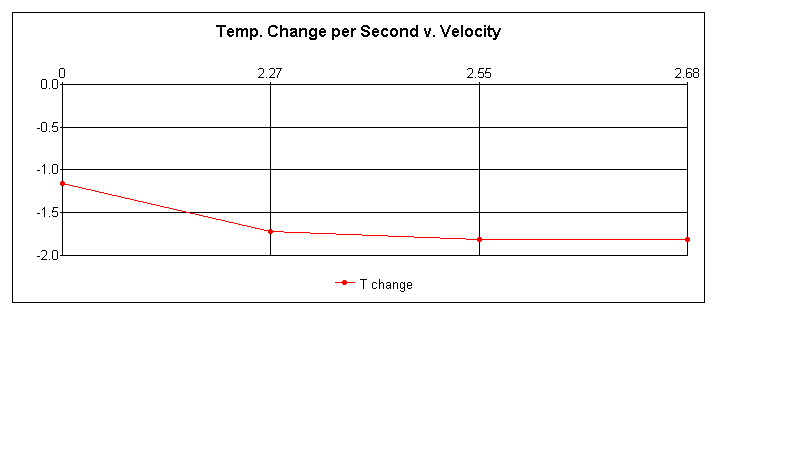
My hypothesis, that the moving water would cause the temperature of the object to decrease more rapidly than if it were simply placed in stale water, and decreasing at a quicker pace in direct accordance with velocity, was partially correct. The moving water did in fact seem to have a significant impact upon the rate of temperature loss; however, despite a slight increase in the cooling rate with quicker water speeds, due to uncertainty, results in this area were inconclusive at best. The minimum and maximum values well overlapped with one another, not to mention a lack of change in rates between 2.55 m/s and 2.68 m/s speeds. A larger range of velocities may have shown this to be otherwise, though doubtful. This experiment could be improved with greater control over outside variants such as viscosity of water, air resistance, air temperature, precision of time, precision of reaction speed, etceteras. This would result in better results and a lack of uncertainty, though this is unlikely to occur unless precise computers are completing the entire experiment in a vacuum. Regardless, I suspect that the results would be similar, with a sharp increase with the addition of a velocity to the water, followed by another sharp decrease in temperature change rates, resulting in a sudden plateau of little or no change, much like an extreme exponential graph.
I would hypothesis this to be the result of a threshold point of sorts, at which entropy is at its full potential and it is impossible for there to be a more rapid rate of temperature change. Let me explain: as the heat moved towards the surface of the object, as is the nature of heat to expand outwards, and as the "blowing" action, which was previously described in the summary of background information in the beginning, increases, it is probable, theoretically, for the heat to be leaving the object via entropy faster than it is able to replenish itself at the surface of the object; thus, it becomes impossible for an increased rate in temperature loss of the object. Again, a threshold at which quicker temperature loss becomes impossible, according to the surface area, the density, and the composition of that object.
In conclusion, I feel rather confident that this experiment succeeded in demonstrating an increased rate of temperature loss according to a velocity of water impacting it, as opposed to being emersed into the water. I also believe that there is a threshold point, as mentioned, and that, contradicting to my hypothesis, temperature change and velocity are not necessary proportional, rather exponential, like the observed temperature drop itself which, at first, drops quickly, only to slow down as it approaches the temperature of the water. These conclusions are only educated guesses, if not merely hunches, though, due to the inconclusiveness of the results.
The Entropy Page - The Entopy Page
Natural Cooling - Various techniques of cooling, and their applications.
Cooling Project - A project involving various forms of cooling, including liquid.
Suzuki Motor Cooling - How circulating/moving liquids (oil) cool an engine.
Some ThermoCourse Page - Lots of brief explanations on various related things.