Viscosity of Corn Syrup and Temperature
Devin Boatsman, Abe Jehnke, Eddy Figueroa, and Andrew Li
Mr. Murray IB Physics Period 06
Table of Contents
Lab Report
The viscosity of High Fructose Corn Syrup over Temperature
Viscosity is essentially how easy or hard it is for a liquid to flow. How does the viscosity of corn syrup change as temperature increases? The independent variable is the temperature of the corn syrup, and the dependent variable is the viscosity of the corn syrup. The controlled variables were the sphere dropped in the corn syrup, temperature of the water, the tube holding the corn syrup, the type of corn syrup, the distance the ball fell, the person timing, the mass of the planet the experiment was conducted on, and the timing device. If we increase the temperature of corn syrup, then the viscosity will decrease because of the attractions between the molecules weakening as the temperature increases.
A. Karo Corn Syrup
B. Tube (Radius .5 cm, height 9 cm)
C. Sphere (Radius 1 mm, mass .0005 kg)
D. Ice
E. Bowl
F. Stove
G. Timer
H. Ruler
I. Water
J. Pot
K. Beaker
L. Cork
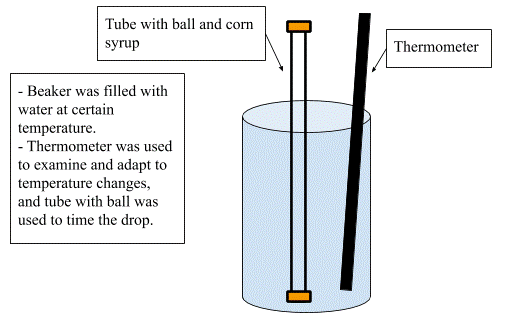
First, gather your materials. Then mark on the tube two lines that are 10 centimeters apart. Fill the tube with cornstarch and place ball inside. Tightly secure tube with cork and try your hardest to ensure that there are no air bubbles. Fill the beaker with water. If you are doing a low temperature, fill beaker half with water, half with ice. If you are doing a hot or warm temperature, fill the beaker with water from the stove. To warm up water, place pot on a stove. Fill the pot with water. Bring water to the desired temperature after turning on the stove. Remove pot filled with water from the stove and turn off the stove.
Place tube with cornstarch and thermometer in the water and wait at least three minutes. Try your hardest to make the water stay at a constant temperature. Make sure the metal ball is at bottom of the tube. When ready fill the tube. Start the timer when the metal ball reaches the black mark. Stop the timer when the ball reaches the second black mark. Record time. Repeat the drops for every trial, and repeat the process from the temperature deciding for every temperature. Now relax, for you have completed an IA lab.
|
Temperature (Degrees Celsius) |
2 |
9 |
23 |
29 |
40 |
51 |
63 |
72 |
78 |
90 |
|
Trial One (s) |
166.06 |
120.32 |
83.40 |
80.59 |
64.05 |
48.56 |
41.61 |
32.28 |
29.58 |
23.49 |
|
Trial Two (s) |
144.87 |
125.52 |
85.36 |
83.85 |
60.59 |
47.91 |
40.80 |
32.44 |
27.51 |
22.91 |
|
Trial Three (s) |
145.93 |
122.91 |
86.83 |
78.26 |
62.42 |
51.76 |
38.49 |
30.92 |
28.97 |
22.35 |
|
Trial Four (s) |
143.82 |
126.73 |
88.24 |
82.37 |
63.26 |
52.61 |
38.89 |
30.16 |
28.15 |
22.16 |
|
Trial Five (s) |
151.84 |
124.13 |
86.22 |
82.23 |
62.84 |
51.62 |
40.21 |
32.64 |
28.81 |
22.99 |
To process the Data we had to combine several different equations in order to find the viscosity of the corn syrup. We put the weight equation (F=mg) equal to the buoyancy (F=pVg) plus Stoke’s law (F=6πnrv), which is mg=pVg+6πnrv, and to get to find viscosity (n) we got n=(mg-pVg)/(6πrv). When we add in the volume of a sphere (V=(4/3)πr^3) for V we get (mg-p(4/3)π(r^3)g)/(6πrv).
|
Temperature |
Viscosity |
|
2 |
435.14±6.08 |
|
9 |
358.29±3.70 |
|
23 |
248.67±2.65 |
|
29 |
235.52±2.70 |
|
40 |
181.08±1.92 |
|
51 |
145.98±1.83 |
|
63 |
115.64±1.38 |
|
72 |
91.618±1.10 |
|
78 |
82.703±0.97 |
|
90 |
65.861±0.71 |
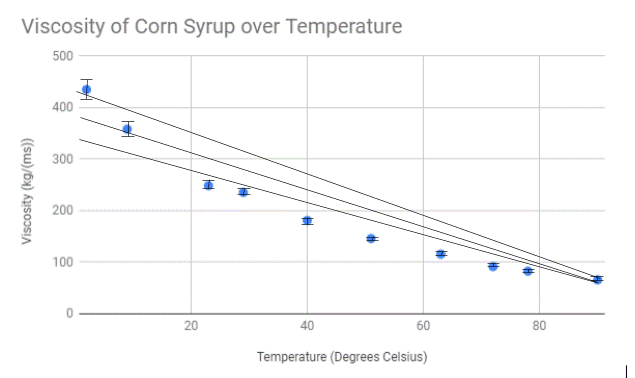
Upon conducting our experiment, we came to the conclusion that increasing the temperature of corn syrup will decrease the viscosity because of the attractions between the molecules weakens as the temperature increases, backing up our hypothesis. This is due to the extra heat in the liquid causing the molecules to become excited and erratic in movement. The energy from the movement is enough to overcome the intermolecular forces and allows the liquid to become more fluid. When the temperature of the corn syrup read two degrees Celcius, the viscosity of the liquid read 435.14 kg/ms. When the temperature read 90 degrees Celsius, the viscosity dropped to 65.861 kg/ms, a significant decrease. In the study by Oluwafunmilayo A. Aworanti, Samuel E. Agarry, and Ayobami O. Ajani, the conclusion states that “The densities and viscosities of binary and ternary blends decreased non-linearly with temperature, respectively”, backing up our claim and expressing the validity of our results.
Simply put, there were a lot of sources of error in our experiment. First off, a large assumption we made was that the temperature of the water would directly go into the temperature of the corn syrup. However, the energy of the temperature likely also went towards the outsides of the container and the direct air above it. It’s impossible for all of the heat energy to transfer into the syrup. There is undoubtedly a temperature difference between the corn syrup in the tube and the water. The water had to influence the glass tube which had to influence the corn syrup. Since it is doubtful that the corn syrup and water were the same temperatures, our viscosity data is likely incorrect to a certain degree. This error is hard to avoid, but we tried our hardest by using a controlled environment and by trying hard to measure the heat loss and minimize it. Also, there is a large source of error in the timing of the ball as it fell. Our timer could not have been correct every time in the start and end of the ball’s descent. Thus, our times are likely off by a small amount. To avoid this error, we had the same person measure the time every time so that their reaction time would be the same more or less for every trial. Another source of error is that the temperature of the water changed over time. In the time between our first and last trials, the temperature of the water would change. Especially if the water was exceptionally cold or warm then it would change dramatically. To avoid this error, we tried to keep water temperatures constant by adding cold or warm water as we went along to manipulate the temperature. Before each trial we measure the water and attempted to keep it as a control throughout the experiment.
In the end, we are fascinated with how corn syrup becomes less viscous in warmer temperatures and more viscous in colder temperatures. The phrases “slow as molasses” and “molasses in January” makes a lot of sense now because the liquid becomes more viscous in the cold temperatures that come with January in the Northern Hemisphere.
https://physics.info/viscosity/
- This source is good because it explains viscosity in depth, and the site explains how to measure viscosity.
https://resources.saylor.org/wwwresources/archived/site/wp-content/uploads/2011/04/Viscosity.pdf
- This source gives graphs and explains the relationship between viscosity and temperature on a molecular level.
http://www.columbia.edu/itc/ldeo/lackner/E4900/Themelis3.pdf
- This source is a textbook on physics from Columbia University that describes viscosity and its varying factors in detail.
- This source first introduces the reader to viscosity, then it gives an exercise that measures viscosity, and there is a informative video on viscosity, too.
https://www.britannica.com/science/viscosity
- This is a good source because it gives a simple encyclopedia definition of viscosity while also providing enough detail to be useful.