The
Affect of Sugar Concentration on the Index of Refraction .:. Go Up
Alexander Dyer
Introduction Hypothesis Scientific Reasoning Independent Variables Dependent Variables Controlled Variables Equipment Diagram of Setup Procedure Method of Analysis Analysis Calculations 1 Angle of Refraction Calculations 2 (Electric Boogaloo) Index of Refraction Calculations 3 (Ron Moholt) Conclusion Limitations Links Citations
Introduction:
Go Up
The goal of
this investigation is to determine the relationship between the density of a
liquid and the index of refraction. Refraction occurs when light goes through
two transparent mediums of different index of refraction values. When light
enters the new medium at any angle, different than perpendicular with the
medium, the light will change its direction, or bend, also known as refraction.
The reason this scientific change in direction of light is interesting to me is
because of the optical illusion side of refraction. I find it incredibly
interesting that an object can appear bigger, or even bent within water, so I
needed to find out why that happened. I would eventually discover that the
reason this optical illusion existed was due to refraction. The reason that
these objects would appear to be bent or bigger was the result of two different
index of refraction values of the two different mediums. Since air and water
have two different index of refraction values, which describes how fast light travels
through the medium, the objects would appear bigger or bent. Knowing how this
illusion works, I furthered my curiosity by wondering how adding sugar to water
would affect the index of refraction for the water. For this investigation, I
decided to determine how sugar concentration in water would affect both the
angle of refraction and index of refraction for water.
Hypothesis:
Go Up
If the sugar concentration within a sample of water is
increased, then the angle of refraction decreases and the index of refraction
increases, because the density shall be increasing do to the sugar
concentration.
Scientific
Reasoning: Go Up
As light passes through different
mediums of different densities, then the angle at which the light is moving
shall change, this is called refraction. This is due to a change in the index
of refraction, so by changing the density of the liquid I am shining a light
into, by adding sugar, then the refractive index should change, which will in
turn make the angle of refraction change with it. Based on Snell’s law, ![]() it’s clear that if index of refraction increases,
then the angle of refraction must decrease, and vice versa. This is clear since
the equation must balance out on both sides, so if one variable increases on
one side, then the other variable on that same side must decrease, in order to
balance the equation.
it’s clear that if index of refraction increases,
then the angle of refraction must decrease, and vice versa. This is clear since
the equation must balance out on both sides, so if one variable increases on
one side, then the other variable on that same side must decrease, in order to
balance the equation.
Independent
Variable: Go Up
The density of the water solution.
Dependent
Variables: The angle of refraction, and index
of refraction.
●
Index of refraction shall be
discover through Snell's Law: ![]()
●
Angle of refraction will be
evaluated through the formula
Controlled
Variables: Go Up
1. Temperature of the medium
a. Reason for control: The temperature of a liquid is a determinant of its density. If the temperature of the medium increases or decreases, then the density will change. This would lead to a change in the index of refraction which would change the the amount that the light bends in the new medium.
b. How to control variable: In order to ensure that the liquid stays at room temperature (20 ± 1 celcius), a thermometer will be used with each new solution of the medium.
2. Angle of Incidence
a.
Reason
for control: The angle at which the light enter
hits the medium will then affect the angle at which the light bends within the
new medium, as can be observed through the formula![]() .
Since a different angle going into the medium will affect the angle at which it
will bend in the medium, a controlled angle will be needed to get the most
accurate results.
.
Since a different angle going into the medium will affect the angle at which it
will bend in the medium, a controlled angle will be needed to get the most
accurate results.
b. How to control variable: This will be accomplished by using a pole and clip to hold a laser pointer at a consistent angle so that the angle will not be subject to change.
Equipment:
Go Up
|
●
Laser pointer ●
1 pole for laser pointer stand ●
Clip to hold laser pointer ●
Meter stick ●
Triple Beam balance |
●
Container that will hold the
liquid ●
Wax paper ●
Sharpie ●
Sugar ●
Measuring cup |
Diagram
of setup: Go Up
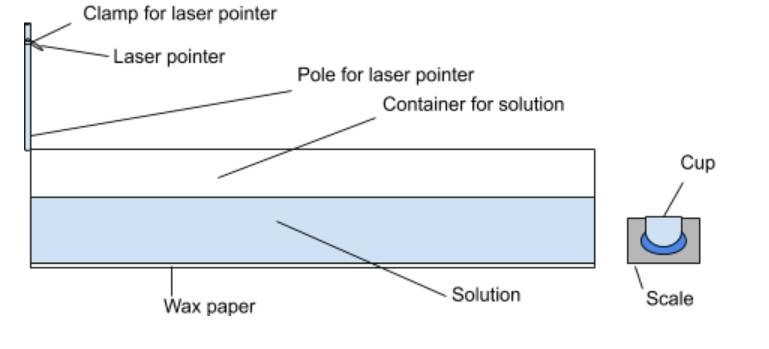

Procedure:
Go Up
The first
step in this investigation was to gather all the necessary equipment and set it
up as dictated in the above diagram. After making sure the equipment was set up
correctly, I would then measure the cup, then add sugar and write down the
values I got from the measurements. I would then subtract the cup mass from the
mass of the sugar and cup, so that I could find out how much sugar I added.
After doing this I would ensure that the temperature was still at room
temperature. I would then make sure all the sugar had dissolved within the
water before I would shine the laser pointer from the pole. Once dissolved, I
would turn on the laser pointer and mark where it hit the bottom of the wax
paper with a sharpie. I would repeat this process for 10 data points, to ensure
the best data possible. After making all the data points in the water, I
removed the wax paper from the bottom of the container and measured the
distances with a meter stick, and noted the measurements for each point on
piece of paper.
How
Refraction was analyzed: Go Up
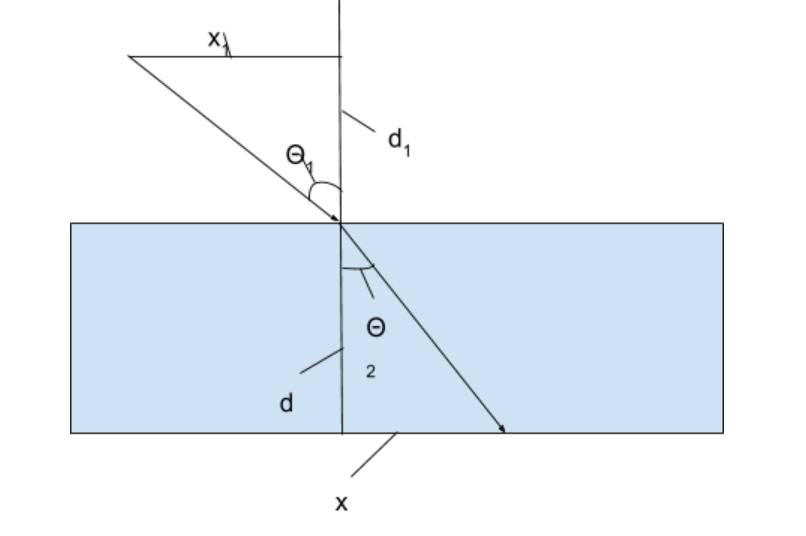

Refraction
patterns were analyzed by measuring the distance the light traveled across the
water, and down. By using these measurements, the angle of refraction could be
discovered and in turn, the index of refraction. There was a sharpie mark where
the light entered the water, and where it hit the bottom of the container. This
was used to find how far the light traveled in the water from the entrance
point. The height of the water in the container was also measured so that the
angle could be found. The angle of incidence was measured through similar means
of measuring the height and distance the laser pointer was from the point it
hit the water.The index of refraction was then
measured by using Snell’s law and using the angles of refraction and incidence
that was observed.
Analysis:
Go Up
Density of the Solutions
At the
start of each new trial, the mass and volume of the solution would be noted as
to find the density through the density formula![]() .
Density is ρ, volume is v, and mass is m. By doing this I was then able to
find the uncertainty of the solution’s density.
.
Density is ρ, volume is v, and mass is m. By doing this I was then able to
find the uncertainty of the solution’s density.
|
Amount of Sugar in the
solution ( |
Mass of the Solution including Cup ( |
Mass of Solution ( m |
Volume of the Solution ( v |
Density (g/mL)
|
Percent Uncertainty |
Density % uncertainty |
|
0 |
1025.4 |
952.6 |
952.6 |
1.000 |
.005774 |
.006 |
|
224.4 |
1249.8 |
1177 |
952.6 |
1.23557 |
.00701 |
.007 |
|
471 |
1496.4 |
1423.6 |
952.6 |
1.49444 |
.008369 |
.008 |
|
749.6 |
1775 |
1702.2 |
952.6 |
1.7869 |
.009904 |
.01 |
|
939.8 |
1965.2 |
1892.4 |
952.6 |
1.98656 |
.010952 |
.011 |
|
1137 |
2162.4 |
2089.6 |
952.6 |
2.19358 |
.012039 |
.012 |
|
1352.1 |
2377.5 |
2304.7 |
952.6 |
2.41938 |
.013224 |
.013 |
|
1538.8 |
2564.2 |
2491.4 |
952.6 |
2.61537 |
.014252 |
.014 |
|
1878.7 |
2904.1 |
2831.1 |
952.6 |
2.97197 |
.016124 |
.016 |
|
2274 |
3299.4 |
3226.6 |
952.6 |
3.38715 |
.018303 |
.018 |
Calculations:
Go Up
The first
step was to determine the mass of the solution, this was accomplished by
subtracting the measuring cup’s mass by the mass of the cup and mass of the
sugar together. By measuring the cup, I determined the mass of the cup was 72.8
![]() .05
g.
.05
g.
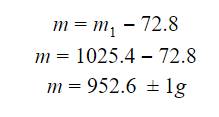
Once the
mass of the liquid was determined, the next calculation was to calculate the
density. To do this I The density of the liquid was determined using the
formula: ![]()
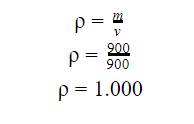
From the volume and the mass, the
total percentage of uncertainty would be evaluated for the density.
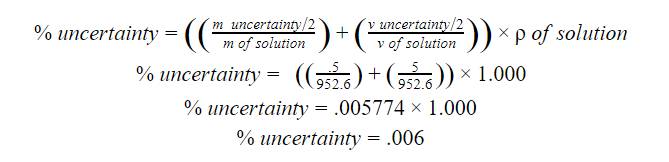
Note: These calculations were repeated
for each amount of sugar added to the overall solution
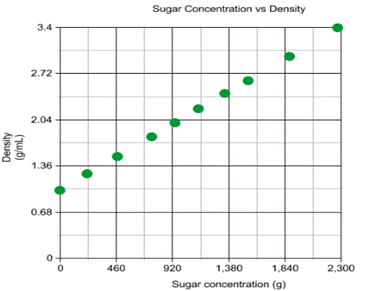
The graph depicts the relationship between the amount of
sugar in the solution, and the density of the solution. Unsurprisingly, as the amount
of sugar increased, the density of the solution also increased. This increase
in density is due to the increase of mass within the same volume, which can be
understood through the formula ![]() .
.
Angle of Refraction Go
Up
The next
step after calculating the density, was to determine the angle of refraction
and the angle of incidence. These were the next calculations to be made in
order to set up for the calculation of the index of refraction.
|
Distance down in air (
|
Distance across in air (
|
Angle of Incidence (
|
Distance down in solution (
|
Distance across in solution (
|
Angle of refraction (
|
|
20 |
32 |
57.99 |
8.9 |
8.4 |
43.34 |
|
20 |
32 |
57.99 |
8.9 |
8.1 |
42.31 |
|
20 |
32 |
57.99 |
8.9 |
8 |
41.95 |
|
20 |
32 |
57.99 |
8.9 |
7.5 |
40.12 |
|
20 |
32 |
57.99 |
8.9 |
7.4 |
39.74 |
|
20 |
32 |
57.99 |
8.9 |
7.3 |
39.36 |
|
20 |
32 |
57.99 |
8.9 |
7.1 |
38.58 |
|
20 |
32 |
57.99 |
8.9 |
6.8 |
37.38 |
|
20 |
32 |
57.99 |
8.9 |
6.5 |
36.14 |
|
20 |
32 |
57.99 |
8.9 |
6.1 |
34.43 |
Calculations:
Go Up
Before I
calculated the angle of refraction, I chose to calculate the angle of
incidence, which was the angle at which it is coming in at the solution. This
calculation was done by using the formula ![]() .
.
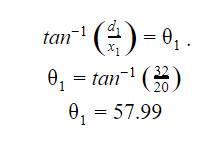
After calculating the angle of
incidence, the next calculation was the angle of refraction, this was
accomplished by using a similar formula that was used for the angle of
incidence, except with using measurements from within the solution![]() .
.
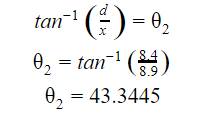
Note: Calculations were repeated for every
new measured distance.
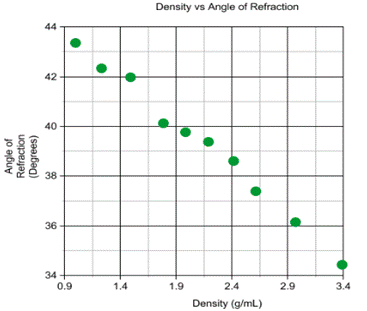
This graph depicts the relationship between the density of
the solution and the angle of refraction. The graph shows that as the density
increased, the angle of refraction would decrease.
Index of Refraction Go
Up
The final calculations for this
investigation was the index of refraction. By calculating this last value, I
would then be able to complete prove or disprove my proposed hypothesis.
|
Index of Refraction for air
|
Angle of Incidence (
|
Angle of Refraction (
|
Index of refraction for solution
|
Index of Refraction for solution %
uncertainty |
|
1.00029 |
57.99 |
43.34 |
1.33838 |
.02798 |
|
1.00029 |
57.99 |
42.31 |
1.37124 |
.02803 |
|
1.00029 |
57.99 |
41.95 |
1.38282 |
.02841 |
|
1.00029 |
57.99 |
40.12 |
1.44592 |
.03049 |
|
1.00029 |
57.99 |
39.74 |
1.45969 |
.03085 |
|
1.00029 |
57.99 |
39.36 |
1.47389 |
.03143 |
|
1.00029 |
57.99 |
38.58 |
1.50362 |
.03245 |
|
1.00029 |
57.99 |
37.38 |
1.55188 |
.03414 |
|
1.00029 |
57.99 |
36.14 |
1.6051 |
.03605 |
|
1.00029 |
57.99 |
34.43 |
1.68508 |
.039 |
Calculations:
Go Up
Finally to
the calculation that was the purpose of this investigation, I calculated the
index of refraction for the solution. In order to calculate the index of
refraction in the solution, I used snell’s law: ![]() .
.
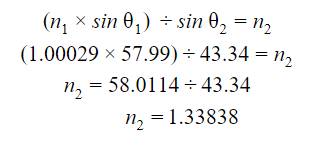
The final
calculation for this investigation was the percent uncertainty in my calculated
index of refraction values. Calculating the % uncertainty in the Index of
Refraction value would use the same uncertainty formula as used for density,
but in terms of the index of refraction.
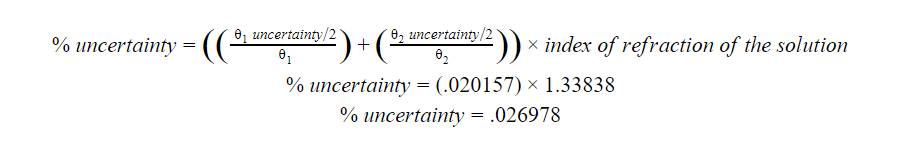
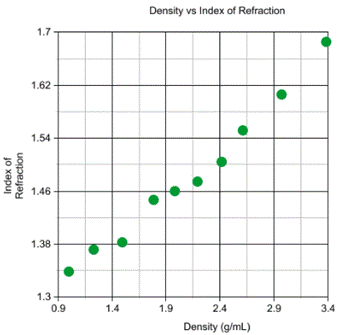
This graph depicts the correlation between the index of refraction and the densities that is
received. This relationship shows that as the density increases, then the angle
of refraction increases as well.
Conclusion Go Up
Based on
the data and calculations I made throughout the investigation, it is evident
that my hypothesis was correct. This investigation was able to prove that if a
substance, such as sugar, is added to water, then the density will increase,
which will also increase the index of refraction value for the water, and also
decrease the angle of refraction. Index of refraction is directly connected to
the density, since as density increases, as does the index of refraction. Based
on this relationship, it can be determined how the index of refraction could be
affected in other substances based on their densities. The denser the medium,
the higher index of refraction value that medium will have. This relationship
also tells a lot about the relationship between density and the angle of
refraction. Since the index of refraction increases with the density, that
makes the angle of refraction decrease with the density. This investigation
ultimately concluded that as density increases index of refraction will
increase, and the angle of refraction will decrease.
Limitations Go Up
One
limitation from this investigation was the thermometer; this could have been a
limitation in the investigation because the thermometer could have been reading
an incorrect temperature. Even though this is an unlikely error that could have
occured in the process of this investigation, it is
possible that it happened. If the thermometer was indeed misread or off, then
it would change the calculations that I got for each variable. Temperature
affects the density of a liquid, with colder temperatures increasing the
density and heat increasing the density. If the temperature was not being told
correctly by the thermometer, then it is possible that the data could have been
skewed by the mistold temperature of the solution. A
solution to this possible error in the investigation is the use of an
electronic thermometer, or the use of multiple thermometer to get multiple
reads on the temperature. By using these methods, this error could reduced in
the investigation.
Another
error that could have occured during the
investigation was residue being added to the water solution that was not
desired, from the wax paper and sharpie. The wax paper was used so that I could
record how far the light travelled horizontally in the water, as was the
sharpie used to notate where light hit the wax paper from the set entrance
point. It's possible that the wax paper would start to leave traces of other
materials in the water. This was a possibility as well when the sharpie was put
in the water to mark where the light was shining. Ink would go into the water
each time it entered. Both of these errors would lead to an increase in
density, which as addressed earlier, would lead to increase in index of refraction
and decrease in angle of refraction. This possible increase in densities would
lead to another possible skew in the data. A possible way to fix this is to use
materials that do not leave residue in the water, or by making the markings on
the outside of the container, so that the water itself is not contaminated.
Another
error that could have occured during the process of
this investigation is if the clip that is holding the laser pointer changed
slightly. If the the clip changed even a little bit, then
the entire data set would have to be to be restarted so that the same angle of
incidence was used for the entire data set. This could be changed by using a
clip that is hard to move, but even with this solution, moving is still a
problem. The best way to deal with this problem, would to measure the distances
that the laser is from the water each time before taking the water
measurements. By doing this, it would ensure that the same angle of incidence s
used the entire time, and it would come to the investigator’s attention when
the angle of incidence changes. With this it would allow the investigator to
know when the data would be no longer valid.
A final
error that could have occured during this
investigation was the assumption that the water being tested was pure water
with no other chemicals with in the water. Granted
that the density calculation showed that it was the density of the water, that
does not mean that there were chemicals in the water that would be added from
the air as time passed on, overall increasing the density. This would also
change the results that would be given through the investigation, making the
results less accurate.A possible way to make the
results more accurate and to eliminate this error, a vacuum chamber would be
optimal. This would ensure that no other micro-objects enter the solution,
leading to a more accurate result.
Links
Go Up
This is a viedoe that helps with understanding refraction and how to
apply Snell’s Law: https://www.khanacademy.org/science/physics/geometric-optics/reflection-refraction/v/refraction-and-snell-s-law
This is a
large index of refraction that helped ensure that my results made some logical
sense: http://hyperphysics.phy-astr.gsu.edu/hbase/Tables/indrf.html
Provided a
small index of refraction to check against other sources and provides other
interesting facts about refraction: https://en.wikipedia.org/wiki/Refractive_index
Another
informative site about refraction, but also has a simulator for refraction,
where you control the index of refraction and the angles: http://hyperphysics.phy-astr.gsu.edu/hbase/geoopt/refr.html
Provided
basic knowledge and understanding of refraction: https://en.wikipedia.org/wiki/Refraction
Citations Go Up
“Refraction.”
Physics: Principles with Applications,
by Douglas C. Giancoli, Pearson Education, 2005, ……..pp. 312–644.
Congratulations,
you found the Ron Moholt Easter egg
I love you Ron Moholt, you are the
best of us and bring light the darkness of the Tualatin Band program. You
always help us achieve our very best, and for that we love you Ron. Thank you
for all the work you do Ron, you will live forever in our hearts.