Corn Syrup: Going the Distance
Ashley Ulberg
Table of Contents: Viscosity Investigation Materials Design Variables Constants Procedure Data Error Conclusion Related Links Appendix Works Cited Return to Research
No two fluids will ever be the same because of all of the variations within their properties. One of the important measurements of a fluid is the resistive flow with the influence of gravity, which is more commonly known as viscosity [1]. This is measured as the total stress of the liquid over the change in the speed of the liquid [2]. Fluids with a higher viscosity slower because it takes a greater amount of force to be able to move than those of a less viscous fluid. Outside of a mere laboratory or classroom setting, viscosity allows people to predict the behaviors of the liquids in order to determine whether they will produce a suitable outcome for a specific goal, such as the correct consistency for glue, baking, and many others. A rather peculiar instance of the importance of viscosity comes from a sticky nightmare on January 15, 1919 in Boston, where a massive burst in a molasses storage tank caused the death of 21 people, many more injuries, and the decimation of a significant portion of the Boston Industrial area [4]. Water, which has a very low viscosity, is able to travel a farther distance in a shorter amount of time [5], unlike molasses on the other hand, which is not able to cover the same amount of ground in as little time. Had the viscosity of the molasses been closer to that of regular water, it is possible much more damage could have been done because of the greater area it could have affected. However, if the molasses was a little higher in viscosity, there could have been a different sticky situation on hand.
Viscosity in This Investigation: Go Up
The purpose of this investigation is to examine how the viscosity of the liquid affects the distance the liquid is able to travel from a hole in a cylinder placed at a constant height. The independent variable is the viscosity of the liquid, while the dependent variable is the horizontal distance the liquid is able to travel.
I believe the results of the investigation will indicate that the distance traveled by the liquid is dependent on the viscosity of the corn syrup solution, where the greater the viscosity, the shorter the distance it flows outward, and the lower the viscosity, the greater the distance traveled is when placed at a constant height above the ground with a hole with a constant radius at a constant height on a constant sized cup.
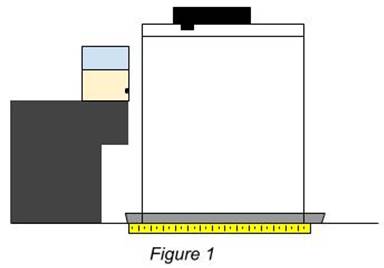
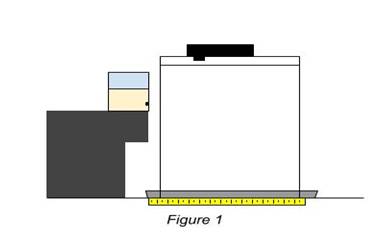
Materials: Go Up
In order to produce the results of this investigation just a few items were needed:
- Distilled water
- Market PantryTM Light Corn Syrup
- Graduated cylinder
- Metric ruler
- Spoon
- Syringe
- Cylindrical glass cup with a hole
- Video camera with slow motion capability
- Tray to catch liquid
Figure above is a representation of the setup used to gather the data for the investigation. The glass with the hole is placed in the counter right above the tray in order to catch the falling liquid. A metric ruler was then also placed within the tray to be lined up directly below the cups hole. A stand is then placed above the tray and cup in order to hold the video camera used to determine the distance the liquid travels.
The independent variable for the investigation is the viscosity of liquid, which is manipulated by creating solutions of the corn syrup by proportionally diluting it with the water.
The dependent variable of the investigation is the distance the liquid is able to travel horizontally from the hole in the glass cup onto the tray below.
The first constant in the investigation is the height at which the glass cup was placed from the counter. The distance of the counter to the hole in the cup is 17.5 centimeters. The same batch of corn syrup was also used to minimize any anomalies. The same cup was also used each time to keep everything consistent.
There is also a constant uncertainty in the measurements of distance and volume. These would be half of the smallest measurement on the instrument, so the distance uncertainty is .05 cm and the uncertainty in volume would be .05 milliliters.
First, the setup was put together and preliminary trials were done in order to ensure the setup was working properly. Next, the first percentage solution was tested in the setup. 200 milliliters of the liquid was poured into the glass with the hole plugged by a syringe. The video was then started and syringe removed. Once the fluid had made contact with the metal tray, the video was stopped and then analyzed in order to determine the exact distance the fluid had traveled horizontally. This was repeated three times for every percentage solution tested. Between each trial, the cup was thoroughly rinsed and dried in order to prevent any remaining solution to skew the results of the following.
The total data collected was from solutions beginning with zero percent corn syrup
and went up by five percent to 75 percent. After all of the data had been
collected, the average of the three tests of each solution variation was found
and then graphed. Figure 2 below is the data in its entirety, including
the average distance which was used in the graph in Figure 3.
|
|
|
Distance (CM) |
|
|
|
% Solution |
Trial 1 |
Trial 2 |
Trial 3 |
Average |
|
0 (Water) |
12.5 |
12.5 |
12.6 |
12.53 |
|
5 |
12.5 |
12.5 |
12.5 |
12.50 |
|
10 |
12.5 |
12.5 |
12.5 |
12.50 |
|
15 |
12.5 |
12.4 |
12.5 |
12.47 |
|
20 |
12.4 |
12.4 |
12.5 |
12.43 |
|
25 |
12.3 |
12.3 |
11.9 |
12.17 |
|
30 |
11.9 |
11.8 |
11.9 |
11.87 |
|
35 |
11.6 |
11.7 |
11.7 |
11.67 |
|
40 |
11.3 |
11.3 |
11.3 |
11.30 |
|
45 |
11.0 |
11.1 |
11.1 |
11.07 |
|
50 |
11.0 |
10.9 |
10.9 |
10.93 |
|
55 |
10.0 |
10.1 |
10.2 |
10.10 |
|
60 |
9.5 |
9.6 |
9.6 |
9.57 |
|
65 |
8.3 |
8.3 |
8.1 |
8.23 |
|
70 |
7.2 |
7.3 |
7.1 |
7.20 |
|
75 |
6.4 |
6.3 |
6.4 |
6.37 |
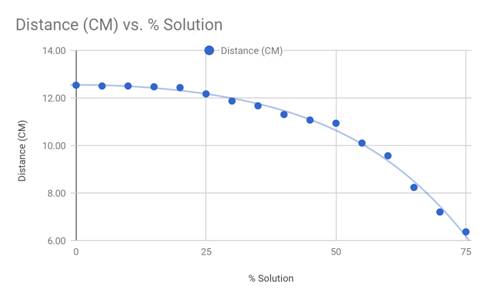
Figure 2
Figure 3
Sources of error from the investigation may include error from the determination of the distance via the video camera. Visual analysis of the video is not extremely exact because of the angle the camera is placed at can skew the perspective of where the liquid hits the ruler. Error can also occur from any change in temperature that may occur from the room itself, changing the viscosity of the corn syrup. This could also happen when the solution was stirred for a longer amount of time.
The results from the investigation confirm
the hypothesis that the higher the percentage of corn syrup solution, the
shorter the distance the fluid was able to travel. Once the averages of the
data were graphed, it is easier to see once the viscosity increases, the
distance traveled decreases on a negative polynomial curve. The line of best
fit seen in Figure 3 is a fourth degree polynomial, which is a fairly
accurate representation of the actual data and relations of viscosity and
distance.
https://www.sciencedaily.com/releases/2016/11/161121090721.htm
This website gives an interesting account of viscosity as a part of a molasses
disaster.
http://www.cscscientific.com/viscosity
This website explains the uses of viscosity.
https://physics.info/viscosity/
This website does a great job of explaining viscosity.
https://www.brookfieldengineering.com/learning-center/learn-about-viscosity/what-is-viscosity
This site gives a more in-depth explanation of viscosity at a higher level.
https://www.saylor.org/site/wp-content/uploads/2011/04/Viscosity.pdf
This PDF gives a great explanation of viscosity, as well as uses, measurements,
and equations.
|
|
|
Distance (CM) |
|
|
|
% Solution |
Trial 1 |
Trial 2 |
Trial 3 |
Average |
|
0 (Water) |
12.5 |
12.5 |
12.6 |
12.53 |
|
5 |
12.5 |
12.5 |
12.5 |
12.50 |
|
10 |
12.5 |
12.5 |
12.5 |
12.50 |
|
15 |
12.5 |
12.4 |
12.5 |
12.47 |
|
20 |
12.4 |
12.4 |
12.5 |
12.43 |
|
25 |
12.3 |
12.3 |
11.9 |
12.17 |
|
30 |
11.9 |
11.8 |
11.9 |
11.87 |
|
35 |
11.6 |
11.7 |
11.7 |
11.67 |
|
40 |
11.3 |
11.3 |
11.3 |
11.30 |
|
45 |
11.0 |
11.1 |
11.1 |
11.07 |
|
50 |
11.0 |
10.9 |
10.9 |
10.93 |
|
55 |
10.0 |
10.1 |
10.2 |
10.10 |
|
60 |
9.5 |
9.6 |
9.6 |
9.57 |
|
65 |
8.3 |
8.3 |
8.1 |
8.23 |
|
70 |
7.2 |
7.3 |
7.1 |
7.20 |
|
75 |
6.4 |
6.3 |
6.4 |
6.37 |
Figure 2
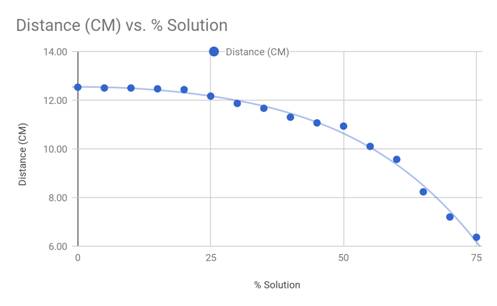
Figure 3
American Institute of Physics. "Molasses Creates a Sticky Situation." ScienceDaily.
November 21, 2016. Accessed October 25, 2017.
https://www.sciencedaily.com/releases/2016/11/161121090721.htm.
Company, CSC Scientific. "Viscosity." What is Viscosity, and Why is Measuring Viscosity
Important? Accessed October 25, 2017. http://www.cscscientific.com/viscosity.
Elert, Glenn. "Viscosity." Viscosity The Physics Hypertextbook. Accessed October 25,
2017. https://physics.info/viscosity/.
"Viscosity." Saylor.org. Accessed October 25, 2017.
https://www.saylor.org/site/wp-content/uploads/2011/04/Viscosity.pdf.
"What is Viscosity?" AMETEK Brookfield. Accessed October 25, 2017.
http://www.brookfieldengineering.com/learning-center/learn-about-viscosity/what-i
[1] Elert, Glenn. "Viscosity." Viscosity The Physics Hypertextbook. Accessed October 25, 2017. https://physics.info/viscosity/.
[2] Company, CSC Scientific. "Viscosity." What is Viscosity, and Why is Measuring Viscosity Important? Accessed October 25, 2017. http://www.cscscientific.com/viscosity.
[3] "What is Viscosity?" AMETEK Brookfield. Accessed October 25, 2017. http://www.brookfieldengineering.com/learning-center/learn-about-viscosity/what-is-viscosity.
[4] American Institute of Physics. "Molasses Creates a Sticky Situation." ScienceDaily. November 21, 2016. Accessed October 25, 2017. https://www.sciencedaily.com/releases/2016/11/161121090721.htm.
[5] "Viscosity." Saylor.org. Accessed October 25, 2017. https://www.saylor.org/site/wp-content/uploads/2011/04/Vis
cosity.pdf.