Viscosity of Corn Syrup Experiment .:. BACK TO RESEARCH POJECTS
Sabian Limones
and Allan George
|Table of Contents|
Question| Hypothesis| Background| Independent Variables| Dependent Variables| Control Variables| Materials| Diagram/Lab Setup| Procedures| Data Tables|
Graphs| Conclusion| Related Sites| Bibliography
Question: How does the dilution of with differing amounts of water affect the viscosity of corn syrup?
Hypothesis: Changing the amount of water to a mixture of corn syrup will make it so the viscosity will increase, with the more amount of water added to the mixture, with the 10 different water dilutions ranging from 0 ml, 2 ml, 4 ml, 6 ml, 8 ml, 10 ml, 12 ml, 14 ml, 16 ml, 18 ml, 20 ml. With the max amount of the mixture will be at 20 ml. The one with the most amount of water added to the mixture will have the fastest time in filling up the beaker to 20ml due to the water having a very low viscosity to it.
Background: The purpose of this lab was to show how different volume levels can affect the viscosity, with this being able to relate to how low viscosity liquid can be changed due to water volume change. This concept of how a change in percentages in a substance with a substance that has a lower viscosity to substances that have a higher viscosity could prove very useful in future experiment involving different substances.
Independent Variables: The percentage of water used to dilute the corn syrup in the experiment with each independent value having a different amount of dilution to it ranging from 0 ml, 2 ml, 4 ml, 6 ml, 8 ml, 10 ml, 12 ml, 14 ml, 16 ml, 18 ml, and 20 ml.
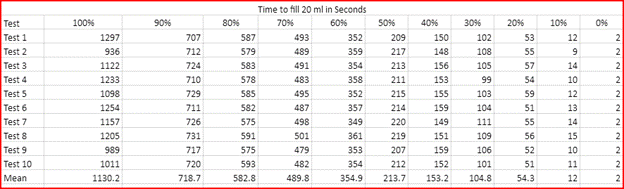
Dependent Variables: The amount of time it takes the beaker to fill to 20 ml with the time starting at the second we start to pore the substance in the beaker.
Control Variables: The room temperature being 64 degrees Fahrenheit and the amount of time a solution was able to sit that being 2 minutes in mix together.
Corn Syrup – The main substance we will be testing to time and use as the control variable
Beaker – The container we will be using to measure the amount of the mixture being poured into and timed
Funnel – Used to help with the pouring process and make sure there is a consistency in it
Water – The other substance we will be testing against the Corn syrup with its pH and temp being around room temp and tap water pH
Graduated Cylinder – The container used to measure out the amount of water used for each experiment to try and keep consistency.
Diagram/Lab setup:
1. The first step is to collect the materials for this lab being a Beaker, Funnel, Graduated Cylinder, a Corn Syrup container, and Water.
2. With the materials you will create the solution with the max amount of solution being 20 ml in the beaker
3. This can be done with first experiment being 20 ml of corn syrup and 0 ml water
4. With the first experiment you will pour the solution in the funnel and start the time.
5. When the solution is poured in, start the time and you will record time when the solution when it fills up to 20 ml
6. This experiment will be conducted 10 times for each for each different solution.
7. This will be conducted with now different percentages being 10%, 20%, 30%, 40%, 50%,60%,70%,80%,90% and 100% water dilution.
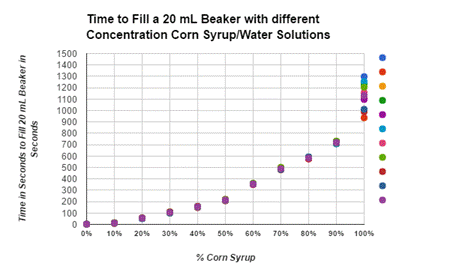
Graphs:
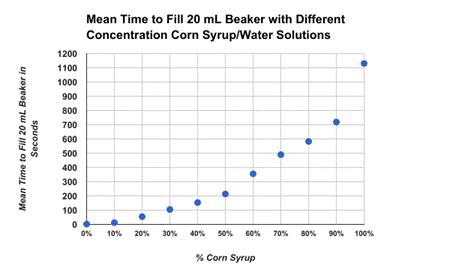
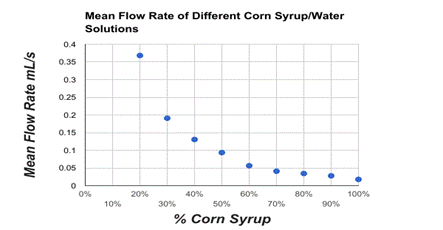
Conclusion:
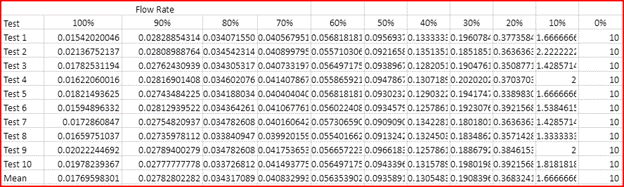
In this lab we were able to show that our hypothesis was correct with the idea that the higher water dilution percentage was the faster the solution was able to fill up 20 ml in the beaker, with the 100% solution of water being the fastest flow rate. The slowest solution was the 0% of water dilution with it having a flow rate of 10 ml/s. This is able to show an exponential decay with the flow rate decreasing with the less water dilution mixed into the solutions. This is able to prove that the dilution of water on corn syrup will increase the flow rate.
< http://trace.tennessee.edu/cgi/viewcontent.cgi?article=2135&context=utk_gradthes > -The research conducted in this experiment gives an example of viscosity and show a different version to this compared to the experiment we conducted.
< https://www.scribd.com/doc/98992432/Full-Report-VISCOUSITY-OF-VISCOUS-FLUIDS > - The research and write up of this paper give example and actual equations we used to come up with our final result for this lab, but also gives its own experiment that was preformed and used these equations.
< https://www.coursehero.com/file/5939952/Viscosity-Report/ > - The website give a lab that can be conducted buy mainly is them racing different liquid and testing there viscosities.
< http://coolscienceexperimentshq.com/viscosity-of-a-liquid-experiment/ >- This website provided a lab write up to an example lab that can be conducted with jars and at home with it also giving good detail to the topic and also providing videos to see how the experiment is conducted and within those videos tells the physics behind the experiment.
Sassin, Ayez. "Full Report VISCOUSITY OF VISCOUS FLUIDS." Scribd. Scribd, n.d. Web. 01 June 2017.
"University
of Tennessee, Research and Creative Exchange." N.p.,
n.d. Web.
"Viscosity
of a Liquid Experiment." Cool Science Experiments Headquarters. N.p., 12 May 2016. Web. 01 June 2017.
"Viscosity
Report - Viscosity Measurement A Report on An..." Viscosity
Report - Viscosity Measurement A Report on an Experiment Performed for ME 120
Experimental. N.p., n.d. Web. 01 June 2017.