The Effect of Different Voltages on the Evaporation Rate of Water
Background.:. Research Question.:. Variables.:. Hypothesis.:. Materials.:. Procedure.:. Data.:. Evaluation.:. Bibliography.:. Related Websites.:. Go Up.:.
Evaporation is the process by which water is converted from its liquid form to gas, often under the influence of heat, (such as a heat lamp). It is necessary for water molecules to have energy in order to evaporate into water vapor. The molecules inside the water have kinetic energy, which are continuously in motion, shifting and colliding with each other. When the molecules collide into each other, there is a transfer of energy between them. The more the molecules collide, the more kinetic energy they have, and the more they collide. Sometimes, when energy is transferred to a molecule near the surface, it is sufficient for the molecule to escape and become water vapor, other times, it is not. Another type of vaporization is boiling, which is characterized by bubbles forming at the surface of the water while the remaining water is still in the liquid state. A few factors that the evaporation rate of water can be influenced by are:
1. The atmospheric pressure surrounding the water:
○ At a lower atmospheric pressure, the evaporation rate of water will be faster than that at a higher atmospheric pressure, because less energy is needed by the water molecules to escape into the atmosphere. Therefore it is easier and quicker for more molecules at the water surface to reach the required energy level necessary to evaporate and become water vapor.
2. The area of the air-water surface:
○ When the surface area of the water is large, the evaporation rate of water will be faster than that of a smaller surface area, because there are significantly more water molecules located near the surface of the water and, therefore, more water molecules will be able to escape into the atmosphere at one time turn into water vapor at a faster rate.
3. The temperature of the surrounding air:
○ Warmer temperatures cause the water molecules to have a greater level of kinetic energy and will produce more collisions between water molecules. This leads to a faster rate of evaporation. This is the same reason that boiling water evaporates quickly.
In a real-world situation of evaporating water, none of the quantities above remains constant because the process of evaporation itself changes them, however in a laboratory experiment, the four are controllable. Water evaporation takes 540 calories per gram of heat away when it evaporates. Thatís enough to cool down 540 grams of water by a degree, or 50 grams of water a little more than ten degrees. If you are not very careful to replace the lost heat energy during the evaporation, the temperature will go down. And even then the temperature right at the surface will be lower than elsewhere in the water and it will depend on water currents convecting heat and the ability to keep the temperature constant at 100 degrees F. For a similar reason, the air near the surface of the water will become more saturated with water as the water evaporates. The evaporation rate will depend on airflow past the water/air surface.
The purpose of this lab is to evaluate how heat affects the rate at which water evaporates when placed under a heat lamp that receives varying voltages.
Dependent:
Mass of evaporated water
Independent:
Various voltages
Control:
Water with no heat applied
If water is placed in a box with a heat lamp suspended above it, then the evaporation rate of the water will increase when more voltage is emitted through a variable transformer, then the heat lamp will emit a higher wattage, which will cause more water to evaporate, because heat causes the evaporation rate of water to increase.
● Shallow Bucket with large surface area
● 50 L of water
● Cardboard box
● Heat lamp with 500 Watt bulb
● Variable Transformer
● Voltmeter
● Scale
● Thermometer
● Timer
In order to begin the
experiment, suspend the heat lamp above the cardboard box. Plug the heat lamp
into the variable transformer. Bring water to room temperature (about 12-13
degrees Celsius). Place container with about 407.3 g of water into the
cardboard box (in order to control air current). Take the temperature of the
water. Leave the water in the box for exactly 15 minutes and then weigh the
water. Take the temperature of the water again. This is the control for the
experiment. For the remaining experiment, vary the voltage the voltage in 10
different increments by using the variable transformer. Take the temperature of
the water before and after. Using a voltmeter, record the voltage that the
variable transformer emits and the current (by putting the leads in parallel
with the wire) for each trial. Run the heat lamp over the water for exactly 15 minutes.
Allow the light bulb to cool between each trial. Measure the remaining mass of
water. Repeat process for 10 trials with each voltage. Calculate the resistance
and power.
|
|
Avg. Voltage +/- 2.6 |
Avg. +/- .2 |
Avg.
+/- .5 |
Avg. Current (A) +/- .28 |
Avg. Resistance ( +/- 2.9 |
Avg. Power (Watts) +/- 2.9 |
|
C |
0 |
4.4 |
0.9 |
0.00 |
0.0 |
0.0 |
|
V1 |
11.0 |
4.6 |
1.3 |
0.00 |
0.0 |
0.0 |
|
V2 |
22.4 |
5.7 |
2.0 |
.95 |
23.6 |
21.3 |
|
V3 |
33.4 |
8.8 |
3.1 |
1.72 |
19.4 |
57.4 |
|
V4 |
45.1 |
12.2 |
4.3 |
2.11 |
21.4 |
95.2 |
|
V5 |
57.3 |
15.5 |
5.2 |
2.45 |
23.4 |
140. |
|
V6 |
69.6 |
19.2 |
10.5 |
2.77 |
25.1 |
193 |
|
V7 |
81.4 |
22.1 |
14.6 |
3.04 |
26.7 |
247 |
|
V8 |
93.0 |
23.8 |
16.0 |
3.31 |
28.1 |
308 |
|
V9 |
104.5 |
27.3 |
23.0 |
3.53 |
29.6 |
369 |
|
V 10 |
116.0 |
28.7 |
31.8 |
3.76 |
30.9 |
436 |
Data file: text
Equations used:
Power: P=IV
Resistance: ![]()
Mean: (Trial 1 + Trial 2 + Trial 3 + Trial 4 + Trial 5 + Trial 6 + Trial 7 + Trial 8 + Trial 9 + Trial 10) / 10
Uncertainty: (((Trial 1 - Average)^2 + (Trial 2 - Average)^2 + (Trial 3 - Average)^2 + (Trial 4 - Average)^2 + (Trial 5 - Average)^2 + (Trial 6 - Average)^2 + (Trial 7 - Average)^2 + (Trial 8 - Average)^2 + (Trial 9 - Average)^2 + (Trial 10- Average)^2)/10)^(Ĺ)
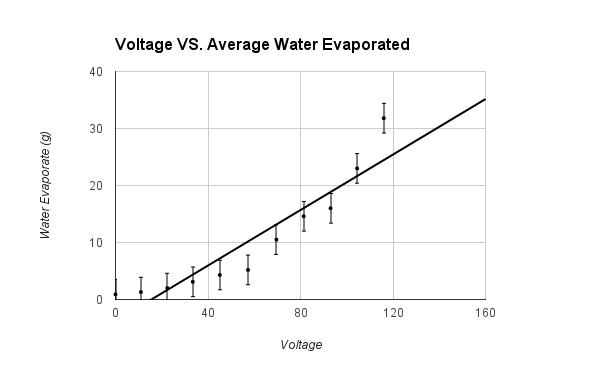
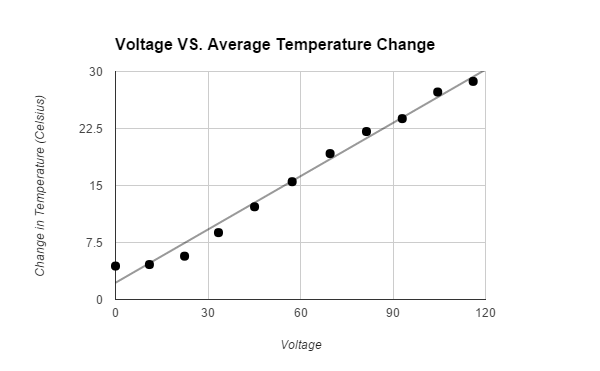
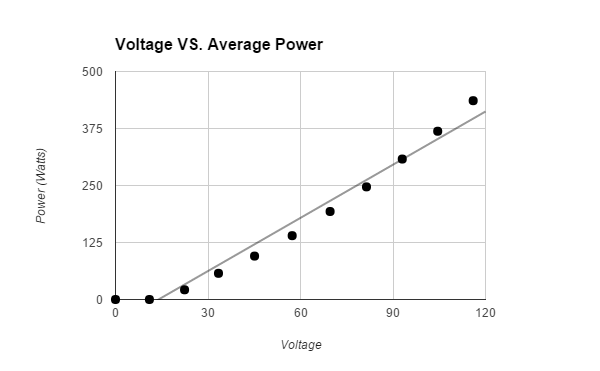
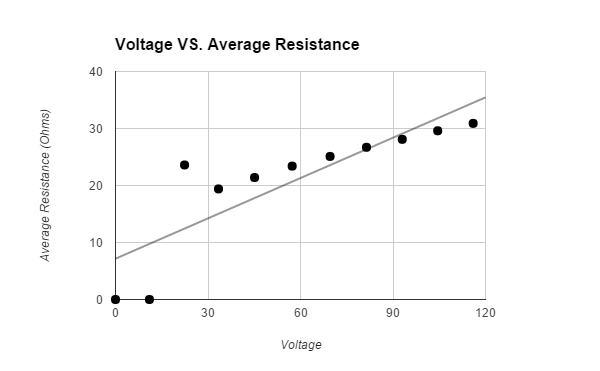
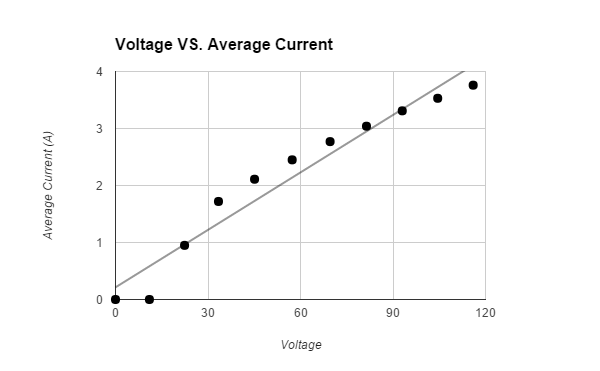
The hypothesis that If water is placed in a box with a heat lamp suspended above it, then the evaporation rate of the water will increase when more voltage is emitted through a variable transformer, then the heat lamp will emit a higher wattage, which will cause more water to evaporate, because heat causes the evaporation rate of water to increase was supported by the data. The data showed that the higher the voltage that the variable transformer emitted, the higher the wattage the light bulb had, therefore the air around the surface of the water was hotter and more water evaporated. All of the trials for the control, that was not exposed to any heat, and first Voltage (about 11.0 V had a Wattage of o.o) had tiny amounts of evaporated water. The second Voltage (about 22.4 V had a Wattage of 21.3) had an average 2.0 +/- .5 g evaporated water. The third Voltage (about 33.4 V had a Wattage of 57.4) had an average 3.1 +/- .5 g evaporated water. The fourth Voltage (about 45.1 V had a Wattage of 95.2) had an average 4.3 +/- .5 g evaporated water. The fifth Voltage (about 57.3 V had a Wattage of 140.) had an average 5.2 +/- .5 g evaporated water. The sixth Voltage (about 69.6 V had a Wattage of 193) had an average 10.5 +/- .5 g evaporated water. The seventh Voltage (about 81.4 V had a Wattage of 247) had an average 14.6 +/- .5 g evaporated water. The eighth Voltage (about 93.0 V had a Wattage of 308) had an average 16.0 +/- .5 g evaporated water. The ninth Voltage (about 104.5 V had a Wattage of 369) had an average 23.0 +/- .5 g evaporated water. The tenth Voltage (about 116.0 V had a Wattage of 436) had an average 31.8 +/- .5 g evaporated water. The data should have come out linearly. It did come out relatively linearly.
There are a few other possible sources of error in this experiment. The experiment took place in a garage instead of a laboratory that would have a controlled temperature. The temperature in the garage fluctuated between trials, and since the overall temperature is a factor in the evaporation rate of water, this could have had a slight effect on the data. Also, the shallow bucket used to hold the water, had a larger surface area than the heat lamp. This means that the center of the bucket had more heat applied to it. This could be fixed by either using a larger heat lamp or a bucket with a smaller surface area.
There are several ways that this experiment could be improved and more in depth for future research. The experiment could be expanded if it were to done twice, one with incandescent bulbs and one with LED lights. The experiment could also be done using different sizes of containers to hold the water in order to discuss how the surface area of the water exposed to air affects the evaporation rate. This experiment has multiple real life applications, one of which being global warming. Another application is for those who own pools, because in order for a pool owner to properly size their dehumidification and ventilation equipment, an accurate calculation of evaporation from the pool is necessary
Evaporation Rate of Water at Room Temperature. (n.d.). Retrieved December 4, 2015.
http://www.edurite.com/kbase/evaporation-rate-of-water-at-room-temperature
Evaporation. (n.d.). Retrieved December 1, 2015.http://techalive.mtu.edu/meec/module01/
EvaporationandTranspiration.htm
Evaporation. (n.d.). Retrieved December 4, 2015.http://www.inquiryinaction.org/classroom
activities/activity.php?id=32
Simplified Method
of Calculating Evaporation From Swimming Pools. (n.d.). Retrieved December 4,
2015. http://hpac.com/humidification-dehumidification/simplified-calculating
-evaporation-1011
Water Evaporation Rate. (n.d.). Retrieved December 4, 2015.https://van.physics.illinois.edu/
qa/listing.php?id=1440.
1. http://www.edurite.com/kbase/evaporation-rate-of-water-at-room-temperature
∑ This is a website discussing the basics of evaporation.
2. http://www.chem4kids.com/files/matter_evap.html
∑ This is helpful, because it is a website that discusses the evaporation of water on a level that a younger audience can understand.
3. http://www.inquiryinaction.org/classroomactivities/activity.php?id=32
∑ This is a website with a similar experiment, for if you would like to check the data of this experiment with others.
4.
http://hpac.com/humidification-dehumidification/simplified-calculating-evaporation-1011
∑ Simple way to calculate the evaporation of water in an area that the results of this study are applicable
5. https://van.physics.illinois.edu/qa/listing.php?id=1440.
∑ This is helpful, because it is a Q & A about water evaporation. It answers the most commonly asked questions.
Go Up.:.