Page #1
The Effect of Temperature on The Bounce of a Tennis Ball.
By: Danh Nguyen & Nick Otto
1/10/15
Per: 3B

Page #2
Table of Contents .:. Top
Page #1: Title
Page #2: Table of Contents
Page #3: Introduction
Page #4: Method
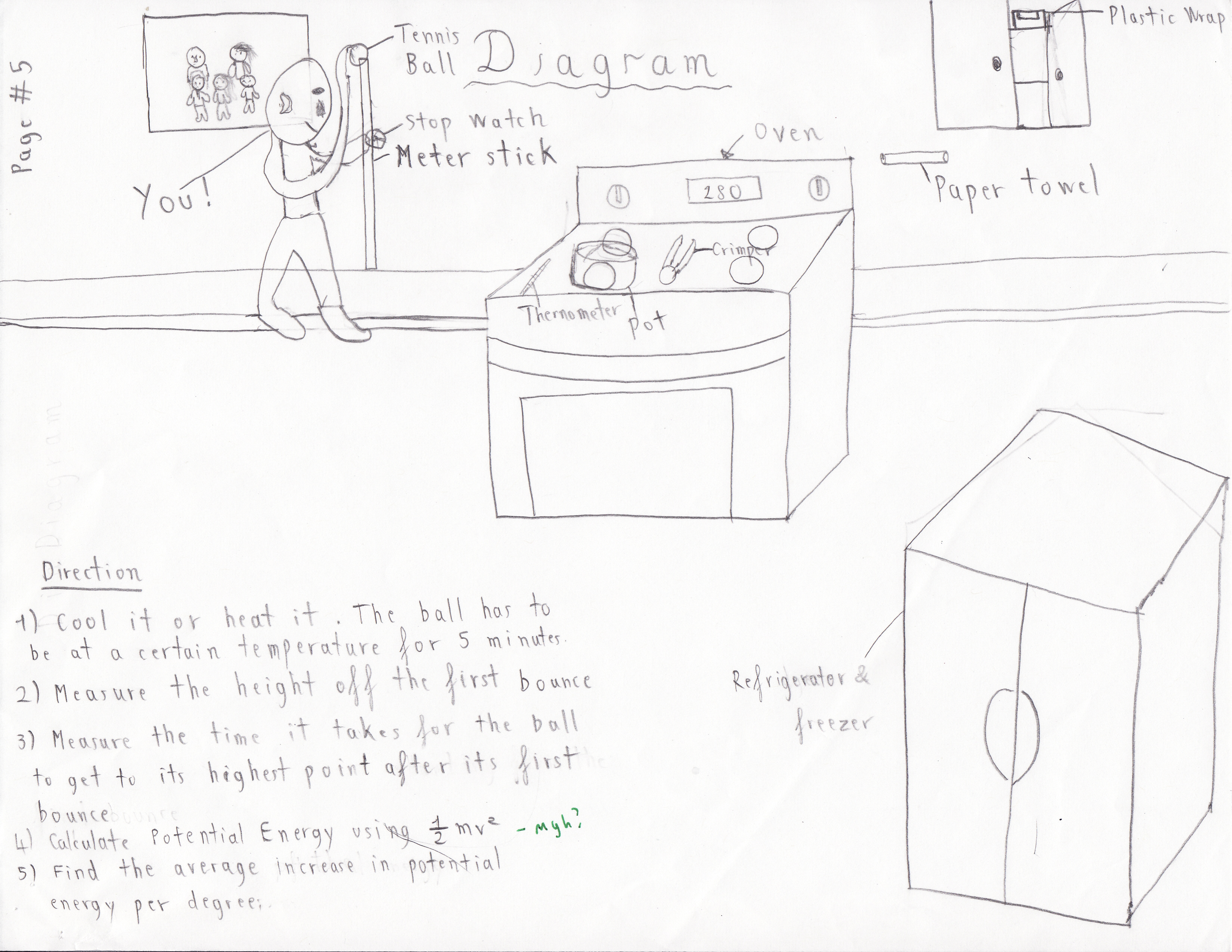
Page #5: Method “Diagram”
Page #6: Results “Data with units and uncertainty”
Page #7: Results “Graphs”
Page #8: Results “General Calculation, Summary of Results”
Page #9: Conclusion
Page #10: Bibliography
LINKS
Page #5: NguyenOttoSetup.jpg
Page #6: IB Physics II project.xlsx
Page #7: index.1.gif
Page #8: NguyenOttoResults.jpg
Page #3
Introduction .:. Top
A lot of people have played some types of sports or activities that involves with a bouncing ball, but many of them do not understand the physics behind it. However, Nick and I will help you understand logic behind it. “We all know that it doesn’t take much to lift a tennis ball off the ground; however, there is work being done to the ball as it is being lifted, giving it energy. We call this energy potential energy” (Energy of a Bouncing Ball). Then, when the ball is dropped the energy is released, and we would call this, kinetic energy. Obviously there is a big relationship between work and energy, the more work the more energy you will get. “Moreover, there is another way you can create more energy by doing the same amount of work. This other form of energy is called temperature.” (The Effect of Temperature on a Bouncing Ball). The reason why temperatures have a big effect on the bounce of the tennis ball is because “the molecules inside the core of the tennis ball is relatively unorganized; they readily slide around and over one another to contract, which can affect the bounce of it” (Sheehan, Krista). This expansion and contract is called pressure. When the temperature increases, the gas molecules inside the tennis ball move faster. As a result, their energy increases when they bounce around more erratically; therefore, this increased temperature results in a higher energy and movement. “On the other hand, a temperature decrease causes the gas molecules to contract and move around more sluggishly. As a result, a cold ball has a much lower bounce” (Energy Transformation in a Bouncing Ball).
From this background information, we hypothesized that if the density and the constant remain the same, the higher temperature will result in higher pressure, while a lower temperature will result in lower pressure. Which mean the ball with the higher pressure will bounce higher, and the one with lower pressure will bounce lower.
During our experiment, well will issued the vertical displacement of the tennis ball to be our independent variable, the height of the ball off the first bounce will be the dependent variable, and at last, the use of the same tennis ball, the same amount of time we leave the ball under a certain temperature, and a woody surface that the ball bounce on to be our control variable.
Independent Variables: vertical displacement of the tennis ball, Temperature (F)
Dependent Variables: the height of the ball of the first bounce (cm)
Control Variables: the same amount of time we leave the ball under a certain temperature (s), the use of the same tennis ball, and a woody surface that the ball bounces on.
Page #4
List of Materials:
● Tennis Ball
● Plastic Wrap
● A pot
● A Crimper
● Fridge
● Oven
● Thermometer
● Meter Stick
● Stopwatch
Direction:
At the beginning of the experiment we had to make a decision on whether we should start measuring the bounce of the ball with a high pressure or a one with a low pressure. So, from the vast knowledge of our physics we determined to start the ball off with a high pressure because the movement of the molecules inside the ball will be more efficient. We imagined that it would be more efficient because the extra energy that it will take to move or get squeezed up won’t be lost. The second steps that we took were measuring the first bounce of the ball and then compare it to the one with the room temperature.
One of the most important criteria we had was no matter what the ball can’t be wet, because if the fur gets wet, the ball will be heavier, which will lead to an inaccurate data. Therefore, when we heated it or cool it, we always try to wrap the ball with a plastic wrap with another layer of paper towel under it, so that the precipitation does not get soaked onto the ball. The second criteria we highly value were how long we left the ball at each temperature. This criteria helps us a lot because even though you leaved the ball at a temperature that is 10 Fahrenheit below the last one, but if it’s left in their longer it has a high potential to reach it’s maximum pressure, while the one that is left in the oven although at higher temperature, but with less amount of time might not have as much pressure.
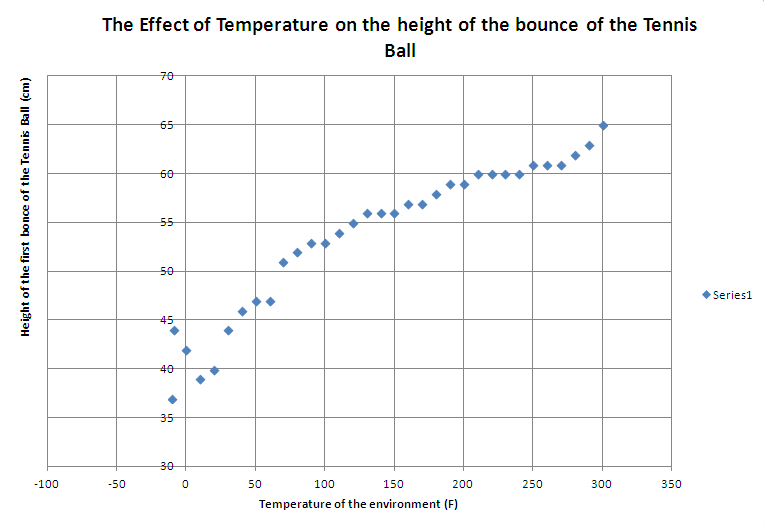
| Temperature of the environment (F) | Height of the first bounce +/- 0.1 (cm) | Mass (g) | Height of the drop (cm) | Difference in Height comparing to Room Temp (+/-) | Time "the amount of time it takes for the ball to reach greatest height of the first bounce +/- 0.001 (s)" | Velocity calculated in (m/s) | Energy calculated in Joules |
| Room Temp | 52 | 5.9 | 100 | 0 | 0.293 | 1.77474 | 0.00929 |
| 300 | 65 | 5.9 | 100 | 13 | 0.333 | 1.95195 | 0.01124 |
| 290 | 63 | 5.9 | 100 | 11 | 0.329 | 1.91489 | 0.01082 |
| 280 | 62 | 5.9 | 100 | 10 | 0.321 | 1.93146 | 0.01101 |
| 270 | 61 | 5.9 | 100 | 9 | 0.328 | 1.85976 | 0.01020 |
| 260 | 61 | 5.9 | 100 | 9 | 0.322 | 1.89441 | 0.01059 |
| 250 | 61 | 5.9 | 100 | 9 | 0.322 | 1.89441 | 0.01059 |
| 240 | 60 | 5.9 | 100 | 8 | 0.319 | 1.88088 | 0.01044 |
| 230 | 60 | 5.9 | 100 | 8 | 0.325 | 1.84615 | 0.01005 |
| 220 | 60 | 5.9 | 100 | 8 | 0.338 | 1.77515 | 0.00930 |
| 210 | 60 | 5.9 | 100 | 8 | 0.311 | 1.92926 | 0.01098 |
| 200 | 59 | 5.9 | 100 | 7 | 0.332 | 1.77711 | 0.00932 |
| 190 | 59 | 5.9 | 100 | 7 | 0.311 | 1.89711 | 0.01062 |
| 180 | 58 | 5.9 | 100 | 6 | 0.300 | 1.93333 | 0.01103 |
| 170 | 57 | 5.9 | 100 | 5 | 0.328 | 1.73780 | 0.00891 |
| 160 | 57 | 5.9 | 100 | 5 | 0.317 | 1.79811 | 0.00954 |
| 150 | 56 | 5.9 | 100 | 4 | 0.309 | 1.81230 | 0.00969 |
| 140 | 56 | 5.9 | 100 | 4 | 0.305 | 1.83607 | 0.00994 |
| 130 | 56 | 5.9 | 100 | 4 | 0.310 | 1.80645 | 0.00963 |
| 120 | 55 | 5.9 | 100 | 3 | 0.302 | 1.82119 | 0.00978 |
| 110 | 54 | 5.9 | 100 | 2 | 0.307 | 1.75896 | 0.00913 |
| 100 | 53 | 5.9 | 100 | 1 | 0.300 | 1.76667 | 0.00921 |
| 90 | 53 | 5.9 | 100 | 1 | 0.303 | 1.74917 | 0.00903 |
| 80 | 52 | 5.9 | 100 | 0 | 0.294 | 1.76871 | 0.00923 |
| 70 | 51 | 5.9 | 100 | -1 | 0.291 | 1.75258 | 0.00906 |
| 60 | 47 | 5.9 | 100 | -5 | 0.282 | 1.66667 | 0.00819 |
| 50 | 47 | 5.9 | 100 | -5 | 0.288 | 1.63194 | 0.00786 |
| 40 | 46 | 5.9 | 100 | -6 | 0.273 | 1.68498 | 0.00838 |
| 30 | 44 | 5.9 | 100 | -8 | 0.297 | 1.48148 | 0.00647 |
| 20 | 40 | 5.9 | 100 | -12 | 0.289 | 1.38408 | 0.00565 |
| 10 | 39 | 5.9 | 100 | -13 | 0.274 | 1.42336 | 0.00598 |
| 0 | 42 | 5.9 | 100 | -10 | 0.284 | 1.47887 | 0.00645 |
| -9 | 44 | 5.9 | 100 | -8 | 0.252 | 1.74603 | 0.00899 |
| -10 | 37 | 5.9 | 100 | -15 | 0.251 | 1.47410 | 0.00641 |

Conclusion .:. Top
In conclusion, with the results we received from our experiment, we were able to concluded and proved that energy is never destroy and in fact, could be transform into a form of heat; or in another word, energy could be created from heat. Along with that, we also accurately hypothesize that if the density and the constant remain the same, the higher temperature will result in higher pressure, while a lower temperature will result in lower pressure. Therefore, when the ball has a lot of pressure to push off of, it will bounce higher than the one with less pressure. Data wise, it was very fluid because all the number seem to match the logic behind what was supposed to happen and through it we were able to calculated the increases in energy per Fahrenheit as the temperature go up to be 1.803 X 10^-5 J/F. From this data, it tell us that as temperature of the ball increases, so does the height of its bounce. We believe this is the case, because when we did the boiling lab in chemistry, we were taught that as temperature increases the molecules of the water starts to move faster and more rapidly, and that change in speed of the molecules is caused from the increased in temperature. In agreement with this, I believe that heating the ball up was exactly the same concept as boiling water because the molecules inside the ball started to move more rapidly creating more energy that can be transfer into heights. It’s just like you throwing the ball at the ground to make it bounce higher; but instead, you don’t have to
do extra work to get that heights that you wanted, all you have to do is heat it up and drop it.
Although we strongly believe that our experiment went fairly well. I also think that we made quite a bit of mistakes. First, would be the timing of the velocity because the pace of the tennis ball bouncing up and down to a naked eye was a little fast, which made us have to guess on when to start and stop the clock. Secondly, getting the data for temperature between 20 to 170 Fahrenheit was extremely difficult because it took a lot of time. After all, we thought the our experiment were super efficient, and if we had to do it over again, the two things we probably would have changed would be the use of a different type of ball. Just to ensure that the ball weight does not get effect if it’s wet so that when we do our calculation, the results that we get matches the correct number. Secondly, testing our experiment with two different balls of the same type to ensure that they reflect the same amount of result.
Bibliography .:. Top
● "The Effect of Temperature on a Bouncing Ball." The Effect of Temperature on a Bouncing Ball. University of Virginia Physics Department, n.d. Web. 28 Oct. 2014.
● Sheehan, Krista. "Does Temperature Affect How High a Tennis Ball Will Bounce?" LIVESTRONG.COM. LIVESTRONG.COM, 06 Feb. 2014. Web. 28 Oct. 2014.
● "Energy of a Bouncing Ball." Energy of a Bouncing Ball. University of Virginia Physics Department, n.d. Web. 06 Nov. 2014.
● "Energy Transformation in a Bouncing Ball - GCSE Science - Marked by Teachers.com." Energy Transformation in a Bouncing Ball - GCSE Science - Marked by Teachers.com. N.p., n.d. Web. 06 Nov. 2014.
RELATED WEBSITES
http://galileo.phys.virginia.edu/outreach/8thGradeSOL/EffectofTemperature.htm
This website is good because it tells the physics behind how to ball bounce higher when its temperature is increase.
http://www.livestrong.com/article/401050-does-temperature-affect-how-high-a-tennis-ball-will-bounce/
This is a good website because it tells you how the tennis is design to bounce.
http://www.gcsescience.com/pen30-energy-ball-bounce.htm
This web page tells you the stages of a bouncing ball.
This is a good web page, because it tells you how you can keep the bounce of the ball efficient.
http://www.123helpme.com/view.asp?id=149221
This is a good web page, because it tells you how others conducted their researches.