Rubber Band Elasticity and Temperature
| Background info | Problem | Review of Literature | Question | Hypothesis | Materials | Procedures |
| Setup Pictures | Data | Graph | Results | Discussion | Return to Research Page | Related Research |
In almost every office around the world you can find a rubber band. Today rubber bands are most commonly used to hold multiple items together. The rubber band’s elasticity, water resistance, and lightness have made it a universal commodity. Modifications to the original rubber band patented in England on March 17, 1845 by Stephen Perry include aerobic bands, bracelets, and projectiles. Close to 21 million tons of rubber were produced in 2005 (“History”). On average, most rubber bands are made out of natural rubber, which performs better under extreme heat than synthetic rubber (Bartleby 2001).
Rubber has been widely used throughout the past era. During World War I Germany created a synthetic rubber to be used in its weapons, but it was too costly. After trade was cut to the East Indies in World War II, which produced most of the United States natural rubber, the Allies decided to begin manufacturing rubber. Synthetic rubber was less costly and continues to account for the majority of the world’s rubber production today.
The purpose of this experiment is to see how the change in temperature affects the elasticity of a rubber band.
While experimenting, we have to keep in mind the different variables involved in the range of elasticity of the rubber band. Vulcanization of the rubber will allow it to be stretched further without breaking (CEC 2007). By making sure the water level is a constant variable we will be able to tell how dependent the rubber bands elasticity is on the change of temperature. When implied force is put on the rubber band an increase and decrease of temperature will show its elasticity effect (McGraw-Hill 2001). To preserve the longest life span of the rubber band when not using it, it’s best to place it in a refrigerator, where no force is put on it. In addition, we must take into account the condition of the rubber band as the temperature increases in order to decrease the probability of error. “Whether a material expands or contracts when it is heated can be ascribed to a property of the material called its entropy” (Shakhashiri). Using the theory on entropy in connection with the elasticity of the rubber band, it can be said that the higher the temperature the higher the entropy level and lower the orderliness of the molecules that make up the rubber. Rubber bands in general stretch more in the presence of heat as Frye states, “rubber contracts when it gets colder. The molecules in the rubber band get closer together. This affects the elasticity…” This means that the band will not be able to stretch as far or support as much weight. We will need to make sure that all these factors remain constant throughout the experiment to present the most valid results.
How does the temperature of a rubber band affect the distance it stretches when submersed in water and supporting a constant weight?
We believe that, as the temperature of the water increases, the rubber band will become more elastic, and therefore it will stretch further. The temperature of the water is the independent variable, and the length of the band is the dependent variable. Knowing that the rubber bands are made out of natural rubber, we think that they will stretch further under warmer temperatures without breaking.
1. One natural Rubber Band
2. Weights
3. 2 wire metals (which are bendable)
4. White board marker
5. One 1000mL beaker
6. Thermometer
7. Paper and pencil
8. Calculator
9. Water/Ice
First, collect the above materials, and find a room at room temperature (about 23° Celsius). The three constants in the lab are the weight, wire, rubber band, and water level. It is important to separately weigh the weights, wires, and rubber band. After weighing, tie the larger wire around the rubber band (see photos below), and with the smaller wire connect the weight onto the other side of the rubber band. Place the rubber band flat on a table and mark two lines from where the rubber band’s elasticity will be measured. Then measure the length of the rubber band within the marks just drawn. Next, observe the 1000ml beaker and convert the distance the rubber band could stretch from ml to cm. Then the experiment can begin. Begin by testing the coldest temperatures. To do this, fill the beaker with ice cubes. Once the ice has melted, put the thermometer in the water to check its temperature (it should be approximately 3° C). Place the apparatus into the beaker once it has reached the right water temperature. Wait a few seconds and observe how far the rubber band has stretched. The elasticity will be measured from the marks drawn on the rubber band. Keep the rubber band submerged in the water as the temperature rises. Every 3° C the water rises, record how far the rubber band stretches. Once the water temperature has reached room temperature take the rubber band out of the beaker. Mark the water level on the beaker after taking the rubber band out. Then, pour out the water and replace it with boiling water. Fill the beaker with boiling water until it reaches the marked line. Place your apparatus back into the beaker and record how far it stretches. Keep the rubber band in as the water temperature decreases. Record how far the rubber band stretches every 3° C change, until it has reached room temperature. Refill beaker with ice cubes and repeat all procedures, as explained above, for two more trials. Finally, plot the data from the three trials and observe how the rubber band's elasticity changed with the different temperatures. By graphing the results, it is easier to determine if the hypothesis was correct or not.
Setup Pictures:

As seen in the pictures, we tied one end of the rubber band to the wire, and the other end was connected to the weights. We then submerged the band and the weights into the water that fills the beaker to 1000mL. We recorded the temperature of the water and measured the length of the rubber band after each interval of temperature dropped or rose (3° C). The length we measured was the distance from the top of the water to where the rubber band was connected to the weights.
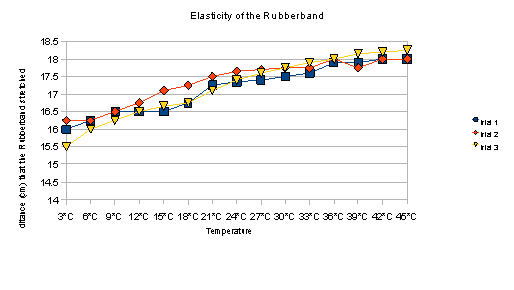
Data:
Constants:
|
Rubber band length |
Weight of the weights |
Weight of the Rubber band |
Weight of the metal device holding the Rubber band |
|
5.5inches |
168grams |
.5grams |
4.6grams |
|
Temperature |
Trial 1 |
Trial 2 |
Trial 3 |
|
3OC |
16cm |
16.25cm |
15.5cm |
|
6OC |
16.25cm |
16.25cm |
16cm |
|
9OC |
16.5cm |
16.5cm |
16.25cm |
|
12OC |
16.5cm |
16.75cm |
16.5cm |
|
15OC |
16.5cm |
17.1cm |
16.65cm |
|
18OC |
16.75cm |
17.25cm |
16.75cm |
|
21OC |
17.25cm |
17.5cm |
17.1cm |
|
24OC |
17.35cm |
17.65cm |
17.4cm |
|
27OC |
17.4cm |
17.7cm |
17.6cm |
|
30OC |
17.5cm |
17.75cm |
17.75cm |
|
33OC |
17.6cm |
17.75cm |
17.9cm |
|
36OC |
17.9cm |
18cm |
18cm |
|
39OC |
17.9cm |
17.75cm |
18.15cm |
|
42OC |
18cm |
18cm |
18.2cm |
|
45OC |
18cm |
18cm |
18.25cm |
Data File (text, tab delimited)
Taking into consideration the fact that we used the same band for repeated trials, the elasticity of the band may have been affected. Aside from this, our data demonstrates that the rubber bands do, in fact, stretch more when submerged in warmer water.
The applications of this study at first seem obscure. But, when viewed in broad terms, it opens questions to how changes in temperature affect specific materials. Although the snap of a rubber band is not usually a cause of worry, the collapse of an operating bridge is. The experiment also points out the major weakness of a rubber band: increased entropy with increased temperature. The question now becomes: how do we build a better rubber band?
Bibliography:
A Handbook for Teachers of Chemistry, Volume 1, by Bassam Z. Shakhashiri, The University of Wisconsin Press, 2537 Daniels Street, Madison, Wisconsin 53704.
Frye, M. (2001, January 31). How does
temperature affect elasticity of a rubber band?
Retrieved October 30, 2007, from <http://www.madsci.org/posts/archives/2001-02/981061637.Eg.r.html>.
“History.” <http://www.therubberband.info/history.php>
McGraw Encyclopedia of Science and Technology. 1960, McGraw-Hill, New
York, New York. Pgs. 672-684.
P. W. Allen, Natural Rubber and the Synthetics (1972); M. Morton, Rubber
Technology
(3d ed. 1987).
“Rubber.” The Columbia Encyclopedia, 6th Ed. New York: Columbia
University Press, 2001–04. <www.bartleby.com/65/>. Oct.2007.
"Secret of Rubber Band." Rubber Bands Specialist. July 2007. Central
Elastic
Corporation. October 28, 2007.<http://72.14.253.104/searchq=cache: hCqiqBxcXoYJ:www.cec.com.my/faq.htm+rubber+band +elasticity&hl=en&ct=clnk &cd=8&gl=us&client=firefox-a>.